Search Thermo Fisher Scientific
Thermo Scientific Chemicals
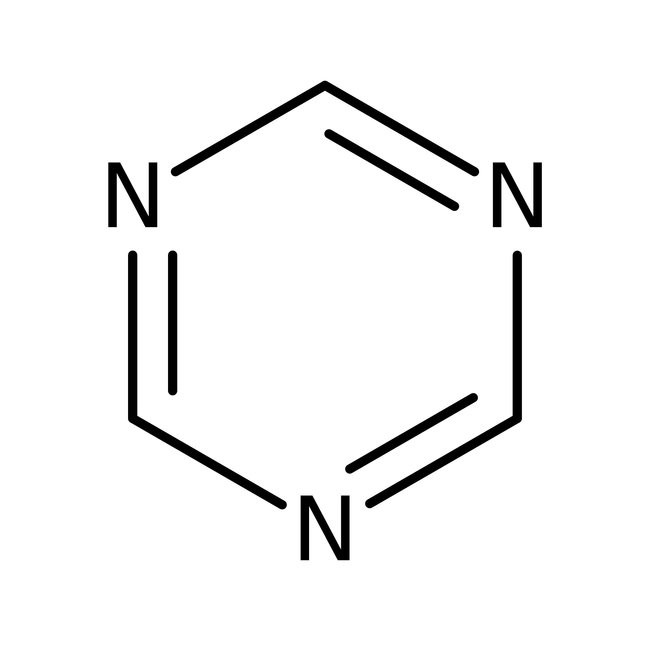
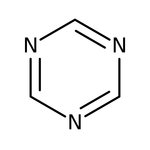
1,3,5-Triazine, 97%, Thermo Scientific Chemicals
CAS: 290-87-9 | C3H3N3 | 81.08 g/mol
Catalog number ALFL16911.03
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
1 g
Specifications
Chemical Name or Material1,3,5-Triazine
CAS290-87-9
Health Hazard 1H302-H315-H318-H335
Health Hazard 2GHS H Statement
H301-H318-H315-H335
Toxic if swallowed.
Causes serious eye damage.
Causes skin irritation.
May cause respiratory irritation.
H301-H318-H315-H335
Toxic if swallowed.
Causes serious eye damage.
Causes skin irritation.
May cause respiratory irritation.
Health Hazard 3P261-P264b-P270-P271-P280-P301+P312-P302+P352-P304+P340-P305+P351+P338-P310-P312-P330-P332+P313-P362-P501c
View more
1,3,5-Triazine is a useful replacement for HCN in the Gattermann reaction for the synthesis of aromatic aldehydes. It reacts with nucleophiles, e.g. amines, which is utilized in quinazoline synthesis.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
1,3,5-Triazine is a useful replacement for HCN in the Gattermann reaction for the synthesis of aromatic aldehydes. It reacts with nucleophiles, e.g. amines, which is utilized in quinazoline synthesis.
Solubility
Soluble in ethanol (50 mg/ml), and methanol (100 mg/ml).
Notes
Store in cool place. Keep container tightly closed in a dry and well-ventilated place. It is hygroscopic in nature. Store under dry inert gas.
1,3,5-Triazine is a useful replacement for HCN in the Gattermann reaction for the synthesis of aromatic aldehydes. It reacts with nucleophiles, e.g. amines, which is utilized in quinazoline synthesis.
Solubility
Soluble in ethanol (50 mg/ml), and methanol (100 mg/ml).
Notes
Store in cool place. Keep container tightly closed in a dry and well-ventilated place. It is hygroscopic in nature. Store under dry inert gas.
RUO – Research Use Only
General References:
- N. G. McCormick.; J. H. Cornell.; A. M. Kaplan. Biodegradation of Hexahydro-1,3,5-Trinitro-1,3,5-Triazine. Appl. Environ. Microbiol.. 1981, 42 (5), 817-823.
- Francis H. Case.; Emil Koft. The Synthesis of Certain Substituted 1,3,5-Triazines Containing the Ferroin Group. J. Am. Chem. Soc. 1959, 81 (4), 905-906.
- Formylating agent: useful replacement for HCN in the Gattermann reaction for the synthesis of aromatic aldehydes: J. Am. Chem. Soc., 76, 290 (1954); Angew. Chem. Int. Ed., 6, 940 (1969); Arch. Pharm., 302, 828 (1969); 304, 362 (1971); with reactive substrates, formylation can be accomplished without a catalyst.
- Also reacts with nucleophiles, e.g. amines, which has been utilized in a quinazoline synthesis: J. Chem. Soc. (C), 1282 (1969):