Search Thermo Fisher Scientific
Thermo Scientific Chemicals
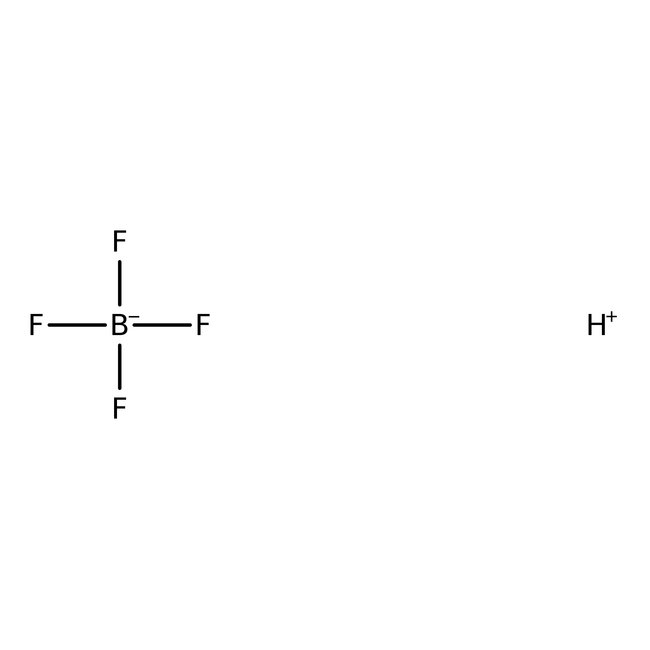
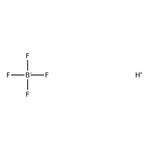
Tetrafluoroboric acid, ca 50% w/w aq. soln., Thermo Scientific Chemicals
CAS: 16872-11-0
| Catalog Number | Quantity |
|---|---|
| ALFL14037.36 | 500 g |
Catalog number ALFL14037.36
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
500 g
Chemical Identifiers
CAS7440-22-4
IUPAC Namesilver
Molecular FormulaAg
InChI KeyBQCADISMDOOEFD-UHFFFAOYSA-N
SMILES[Ag]
View more
Tetrafluoroboric acid is used in conjunction with various methods of cleaning and to treat the surface of substrates. It is also used as a radiotracer.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Tetrafluoroboric acid is used in conjunction with various methods of cleaning and to treat the surface of substrates. It is also used as a radiotracer.
Solubility
Miscible with water.
Notes
Incompatible with strong bases, oxidizing agents and acids.
Tetrafluoroboric acid is used in conjunction with various methods of cleaning and to treat the surface of substrates. It is also used as a radiotracer.
Solubility
Miscible with water.
Notes
Incompatible with strong bases, oxidizing agents and acids.
RUO – Research Use Only
General References:
- Precursor of tetrafluoroborate salts of various stabilized cations. See, e.g.: Org. Synth. Coll., 5, 1135 (pyrylium), 1138 (tropylium); 6, 364 (sulfonium), 991 (cyclopropenium).
- Addition to aqueous solutions of aryldiazonium salts usually results in precipitation of the diazonium tetrafluoroborates, which are often stable enough to be isolated, and can undergo various reactions in non-aqueous solvents. For hydrodediazoniation in the presence of formamide, see: J. Chem. Soc., Perkin 1, 873 (1986). Probably their most important use is the Schiemann (or Balz-Schiemann) synthesis of aryl fluorides in which the tetrafluoroborate salt is heated neat or in an inert solvent. For examples, see: Org. Synth. Coll., 2, 188, 295, 299 (1943). Reviews: Org. React., 5, 193 (1949); Adv. Fluorine. Chem., 4, 1 (1965). See also Hexafluorophosphoric acid, L15728.
- Chen, L.; Cao, X.; Huang, D.; Pan, Q. Switchable dielectric phase transition in tris(1-(chloromethyl)-1,4-diazabicyclo[2.2.2]octane) tetra(tetrafluoroborate) dichloride. Inorg. Chem. Commun. 2015, 61, 93-96.
- Zhong, Z.; Brokmeiera, H.; Maawad, E.; Schell, N. In-situ investigation of the anisotropic mechanical behavior of rolled AA 7020-T6 alloy through lattice strain evolution during uniaxial tension. Mater. Sci. Eng., A 2015, 639, 519-525.