Search Thermo Fisher Scientific
Thermo Scientific Chemicals
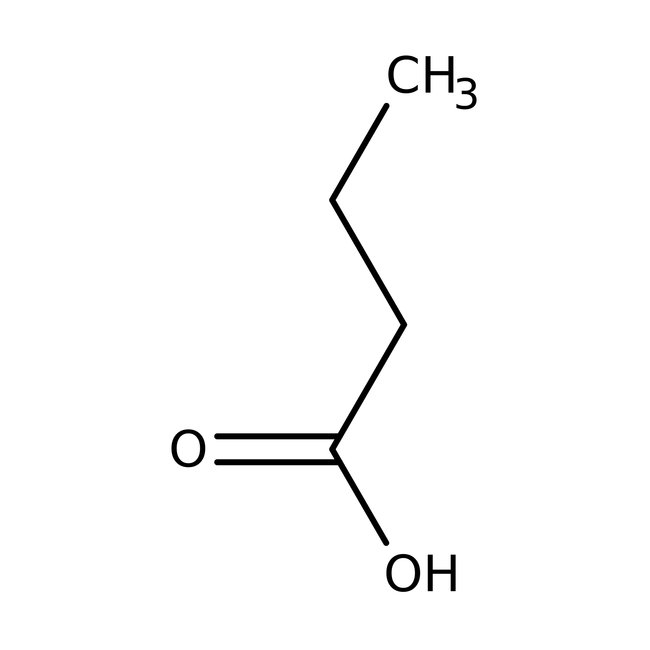
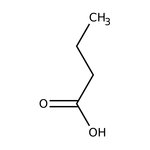
Butyric acid, 99+%, Thermo Scientific Chemicals
CAS: 107-92-6 | C4H8O2 | 88.11 g/mol
Catalog number ALFL13189.AE
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
100 mL
Chemical Identifiers
CAS54-85-3
IUPAC Namepyridine-4-carbohydrazide
Molecular FormulaC6H7N3O
InChI KeyQRXWMOHMRWLFEY-UHFFFAOYSA-N
SMILESNNC(=O)C1=CC=NC=C1
View more
Specifications Specification Sheet
Specification Sheet
Appearance (Form)Powder or crystals
Titration with HClO4>=98.5 % (On dried substance)
Melting point170°C to 174°C
Appearance (Color)Colorless to white to off-white
Infrared spectrumConforms
View more
Butyric acid is a useful as precursor for the preparation of various butyrate esters. It is used as a food and perfume additive. It is also used as an animal feed supplement. Due to its powerful odor, it has also been used as a fishing bait additive and as a stink bomb. It is widely used as a flavoring agent for human food and beverages.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Butyric acid is a useful as precursor for the preparation of various butyrate esters. It is used as a food and perfume additive. It is also used as an animal feed supplement. Due to its powerful odor, it has also been used as a fishing bait additive and as a stink bomb. It is widely used as a flavoring agent for human food and beverages.
Solubility
Miscible with water, ethanol and ether. Slightly miscible with carbon tetrachloride.
Notes
Store in cool place. Incompatible with strong oxidizing agents.
Butyric acid is a useful as precursor for the preparation of various butyrate esters. It is used as a food and perfume additive. It is also used as an animal feed supplement. Due to its powerful odor, it has also been used as a fishing bait additive and as a stink bomb. It is widely used as a flavoring agent for human food and beverages.
Solubility
Miscible with water, ethanol and ether. Slightly miscible with carbon tetrachloride.
Notes
Store in cool place. Incompatible with strong oxidizing agents.
RUO – Research Use Only
General References:
- Ching, S.; Richter, I. J.; Tutunjian, K. A.; Kriz, D. A.; Kovic, Y. Synthesis of highly monodisperse porous manganese oxide spheres using a butyric acid microemulsion. Chem. Commun. 2015, 51 (10), 1961-1964.
- Bianchi, N.; Chiarabelli, C.; Zuccato, C.; Lampronti, I.; Borgatti, M.; Amari, G.; Delcanale, M.; Chiavilli, F.; Prus, E.; Fibach, E.; Gambari, R. Erythroid differentiation ability of butyric acid analogues: Identification of basal chemical structures of new inducers of foetal haemoglobin. Mol. Cell. Pharm. 2015, 752, 84-91.