Search Thermo Fisher Scientific
Thermo Scientific Chemicals
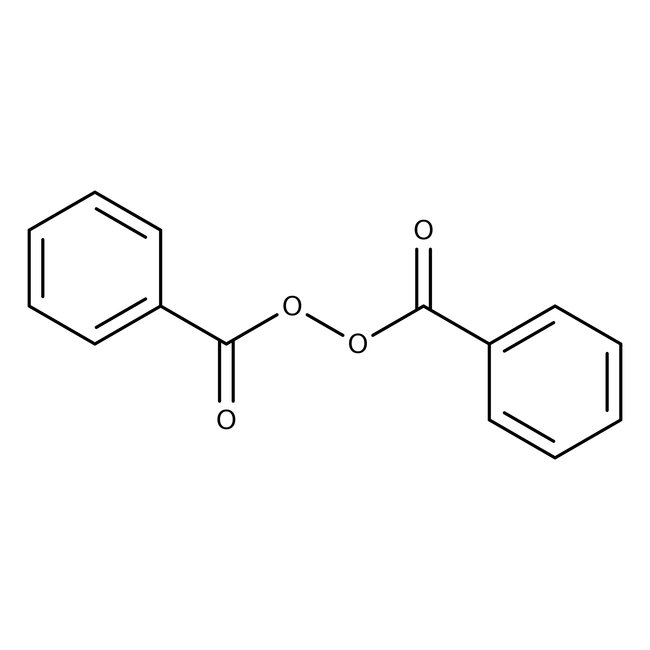
Dibenzoyl peroxide, 97% (dry wt.), wet with 25% water, Thermo Scientific Chemicals
CAS: 94-36-0 | C14H10O4 | 242.23 g/mol
Catalog number ALFL13174.30
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
250 g
Specifications
Chemical Name or MaterialBenzoyl peroxide
CAS94-36-0
Health Hazard 1H242-H317-H319-H335
Health Hazard 2GHS H Statement
H242-H319-H317
Heating may cause a fire.
Causes serious eye irritation.
May cause an allergic skin reaction.
H242-H319-H317
Heating may cause a fire.
Causes serious eye irritation.
May cause an allergic skin reaction.
Health Hazard 3P210-P220-P234-P261-P264b-P271-P272-P280-P302+P352-P304+P340-P305+P351+P338-P312-P333+P313-P363-P501c
View more
Benzoyl peroxide is widely utilized as a radical initiator to induce polymerizations. It finds applications for acne treatment, for bleaching flour, hair and teeth and for cross-linking polyester resins. It also has major applications in antiseptic and bleaching properties. It serves as a catalyst for polyester thermoset resins and as a hardener to start the polymerization process.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Benzoyl peroxide is widely utilized as a radical initiator to induce polymerizations. It finds applications for acne treatment, for bleaching flour, hair and teeth and for cross-linking polyester resins. It also has major applications in antiseptic and bleaching properties. It serves as a catalyst for polyester thermoset resins and as a hardener to start the polymerization process.
Solubility
Soluble in ether and chloroform. Slightlysoluble in ethanol. Insoluble in water.
Notes
Incompatible with strong oxidizer, reducing agents, acids, bases, alcohols and metals.
Benzoyl peroxide is widely utilized as a radical initiator to induce polymerizations. It finds applications for acne treatment, for bleaching flour, hair and teeth and for cross-linking polyester resins. It also has major applications in antiseptic and bleaching properties. It serves as a catalyst for polyester thermoset resins and as a hardener to start the polymerization process.
Solubility
Soluble in ether and chloroform. Slightlysoluble in ethanol. Insoluble in water.
Notes
Incompatible with strong oxidizer, reducing agents, acids, bases, alcohols and metals.
RUO – Research Use Only
General References:
- Free-radical initiator for a wide variety of homolytic reactions, e.g. halogenations with bromine or NBS.
- Semsarzadeh, M. A.; Alamdari, P. Co(acac)2 mediated controlled radical copolymerization of vinyl acetate and methyl acrylate initiated by benzoyl peroxide. Macromol. Res. 2015, 23 (2), 139-144.
- Gollnick, H. P. M.; Friedrich, M.; Peschen, M.; Pettker, R.; Pier, A.; Jostingmeyer, V. S.; Porombka, D.; Pulido, R.; Jackel, A. Safety and efficacy of adapalene 0.1% / benzoyl peroxide 2.5% in the long-term treatment of predominantly moderate acne with or without concomitant medication - results from the non-interventional cohort study ELANG. J Eur Acad Dermatol Venereol 2015, 29 (4), 15-22.