Search Thermo Fisher Scientific
Thermo Scientific Chemicals
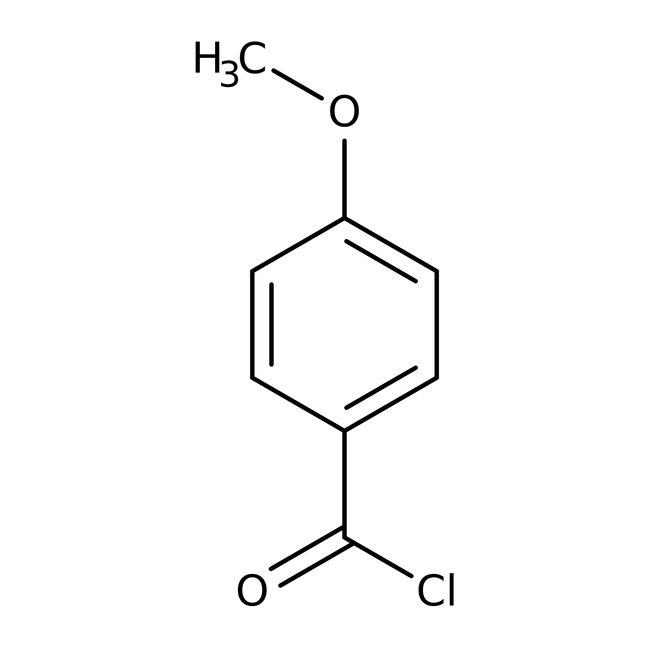
4-Methoxybenzoyl chloride, 97%, Thermo Scientific Chemicals
CAS: 100-07-2 | C8H7ClO2 | 170.59 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFL13120.14 | 25 g |
Catalog number ALFL13120.14
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
25 g
Chemical Identifiers
CAS98-55-5
IUPAC Name2-[(1R)-4-methylcyclohex-3-en-1-yl]propan-2-ol
Molecular FormulaC10H18O
InChI KeyWUOACPNHFRMFPN-VIFPVBQESA-N
SMILESCC1=CC[C@@H](CC1)C(C)(C)O
View more
Specifications Specification Sheet
Specification Sheet
Infrared spectrumConforms
Appearance (Form)Viscous liquid or low melting crystalline mass or
Refractive index1.4800 to 1.4850 (20°C, 589 nm)
Appearance (Color)Clear colorless to white
GC>=97.0 %
View more
4-Methoxybenzoyl chloride is used in the synthesis of stilbene and dihydrostilbene derivatives as potential anti-cancer agents. It is also used in the synthesis of coumarin dimers with potential HIV-1 activity.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
4-Methoxybenzoyl chloride is used in the synthesis of stilbene and dihydrostilbene derivatives as potential anti-cancer agents. It is also used in the synthesis of coumarin dimers with potential HIV-1 activity.
Solubility
It reacts in water.
Notes
Moisture sensitive. Incompatible with oxidizing agents and bases.
4-Methoxybenzoyl chloride is used in the synthesis of stilbene and dihydrostilbene derivatives as potential anti-cancer agents. It is also used in the synthesis of coumarin dimers with potential HIV-1 activity.
Solubility
It reacts in water.
Notes
Moisture sensitive. Incompatible with oxidizing agents and bases.
RUO – Research Use Only
General References:
- Martyn J.Earle; Paul B.McCormac; Kenneth R.Seddon. The first high yield green route to a pharmaceutical in a room temperature ionic liquid. Green Chem. 2000, 2,(6), 261-262.
- Stephen T.Heller; Swaminathan R.Natarajan. 1,3-Diketones from acid chlorides and ketones: A rapid and general one-pot synthesis of pyrazoles. Org. Lett. 2006, 8,(13), 2675-2678.