Search Thermo Fisher Scientific
Thermo Scientific Chemicals
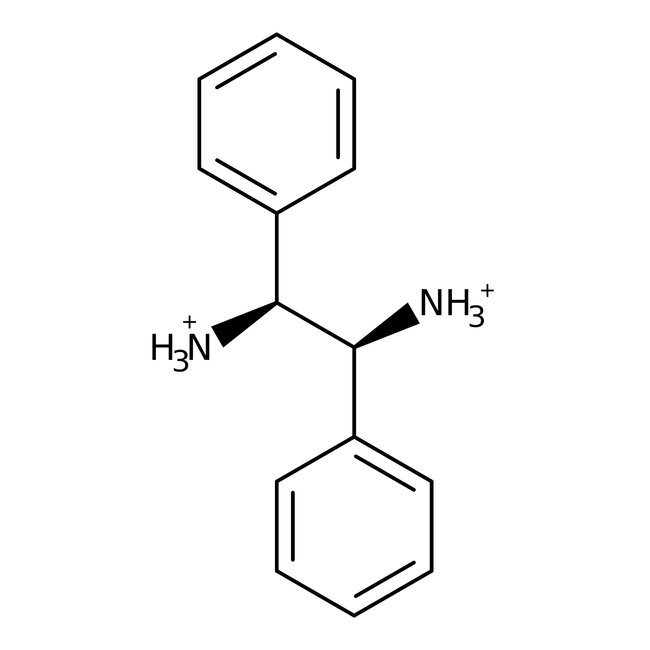
(1S,2S)-(-)-1,2-Diphenyl-1,2-ethanediamine, 97%, Thermo Scientific Chemicals
CAS: 29841-69-8 | C14H16N2 | 212.296 g/mol
Catalog number ALFL12968.MD
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
250 mg
Specifications
Chemical Name or Material(1S,2S)-(-)-1,2-Diphenyl-1,2-ethanediamine
CAS29841-69-8
Health Hazard 1H302+H312-H314
Health Hazard 2GHS H Statement
H314-H318
Causes severe skin burns and eye damage.
Causes serious eye damage.
H314-H318
Causes severe skin burns and eye damage.
Causes serious eye damage.
Health Hazard 3P260-P264b-P270-P271-P280-P303+P361+P353-P304+P340-P305+P351+P338-P310-P330-P331-P363-P501c
View more
(1S,2S)-(-)-1,2-Diphenyl-1,2-ethanediamine solvation agent, For synthesis of enantiopure ethylenediamines by chirality transfer (condensation with diketones followed by reductive cleavage), Co-catalyst in the Ru catalyzed enantioselective hydrogenation of aromatic ketones, Versatile ligand for the formation of metal complexes.1 Used in the synthesis of chiral tropocoronands which have potential utility in asymmetric catalysis
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
(1S,2S)-(-)-1,2-Diphenyl-1,2-ethanediamine solvation agent, For synthesis of enantiopure ethylenediamines by chirality transfer (condensation with diketones followed by reductive cleavage), Co-catalyst in the Ru catalyzed enantioselective hydrogenation of aromatic ketones, Versatile ligand for the formation of metal complexes.1 Used in the synthesis of chiral tropocoronands which have potential utility in asymmetric catalysis
Solubility
Insoluble in water.
Notes
Keep container tightly closed. Store in cool, dry conditions in well sealed containers. It is sensitive to air.
(1S,2S)-(-)-1,2-Diphenyl-1,2-ethanediamine solvation agent, For synthesis of enantiopure ethylenediamines by chirality transfer (condensation with diketones followed by reductive cleavage), Co-catalyst in the Ru catalyzed enantioselective hydrogenation of aromatic ketones, Versatile ligand for the formation of metal complexes.1 Used in the synthesis of chiral tropocoronands which have potential utility in asymmetric catalysis
Solubility
Insoluble in water.
Notes
Keep container tightly closed. Store in cool, dry conditions in well sealed containers. It is sensitive to air.
RUO – Research Use Only
General References:
- Takeshi Ohkuma.; Hirohito Ooka.; Shohei Hashiguchi.; Takao Ikariya.; Ryoji Noyori. Practical Enantioselective Hydrogenation of Aromatic Ketones. J. Am. Chem. Soc.. 1995, 117 (9), 2675-2676.
- Michael H. Nantz.; David A. Lee.; Daniel M. Bender.; Azeen H. Roohi. An enantioselective synthesis of vicinal diamines. J. Org. Chem. 1992, 57 (24), 6653-6657.
- Chiral auxiliary and solvation agent; see preceding entry.
- For synthesis of enantiopure ethylenediamines by chirality transfer (condensation with diketones followed by reductive cleavage), see: J. Org. Chem ., 57, 6653 (1992).
- Co-catalyst in the Ru catalyzed enantioselective hydrogenation of aromatic ketones: J. Am. Chem. Soc., 117, 2675 (1995).