Search Thermo Fisher Scientific
Thermo Scientific Chemicals
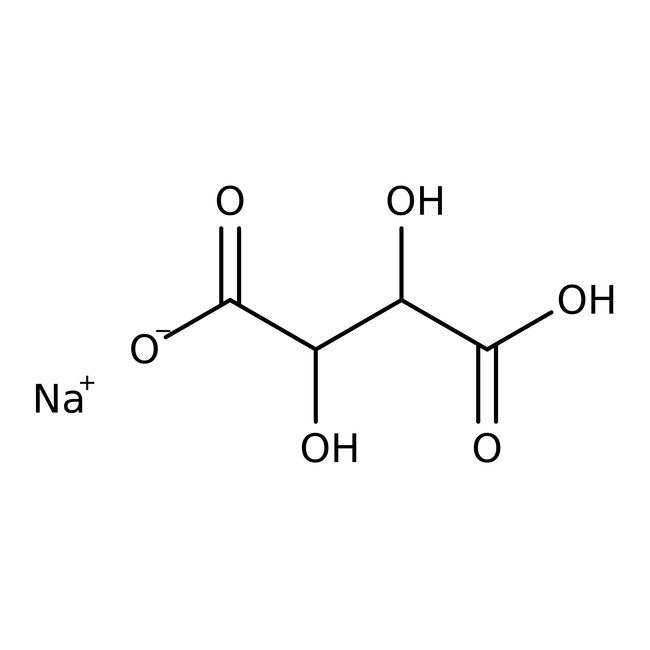
Sodium hydrogen L-tartrate, anhydrous, 98%, Thermo Scientific Chemicals
CAS: 526-94-3 | C4H5NaO6 | 172.07 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFL12469.22 | 100 g |
Catalog number ALFL12469.22
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
100 g
Chemical Identifiers
CAS575-38-2
IUPAC Namenaphthalene-1,7-diol
Molecular FormulaC10H8O2
InChI KeyZUVBIBLYOCVYJU-UHFFFAOYSA-N
SMILESOC1=CC=C2C=CC=C(O)C2=C1
View more
Specifications Specification Sheet
Specification Sheet
Appearance (Color)white to yellow, grey or dark brown
FormPowder
Assay (GC)> 96.0%
Proton NMRConforms to structure
Sodium hydrogen L-tartrate is used for detecting potassium and in nutrient media. It also used as a food additive and an acidity regulator.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Sodium hydrogen L-tartrate is used for detecting potassium and in nutrient media. It also used as a food additive and an acidity regulator.
Solubility
Soluble in water.
Notes
Incompatible with strong oxidizing agents.
Sodium hydrogen L-tartrate is used for detecting potassium and in nutrient media. It also used as a food additive and an acidity regulator.
Solubility
Soluble in water.
Notes
Incompatible with strong oxidizing agents.
RUO – Research Use Only
General References:
- Charoen-In, U.; Ritjareonwattu, S.; Pairwatthanaphaisan, P.; Manyum, P. Crystal Growth, Structural, Thermal, Optical, Dielectric, and Ferroelectric Characterization of Sodium Hydrogen L-Tartrate Single Crystals. Integr. Ferroelectr. 2015, 165 (1), 1-10.
- Bartek, L.; Klusoň, P.; Červený, L. In situ chiral modification of nickel catalysts for enantioselective hydrogenation of methyl acetoacetate. Collect. Czech. Chem. Commun. 2005, 70 (10), 1642-1652.