Search Thermo Fisher Scientific
Thermo Scientific Chemicals
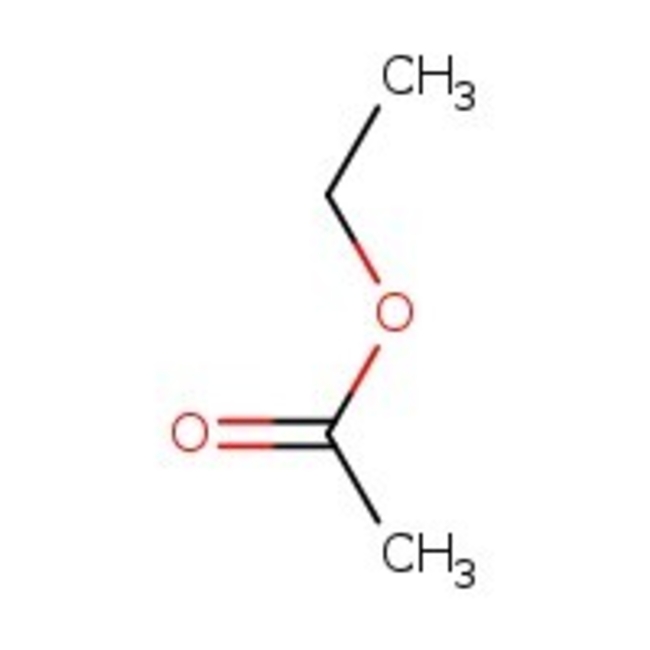
Ethyl acetate, 99%, Thermo Scientific Chemicals
CAS: 141-78-6 | C4H8O2 | 88.106 g/mol
Catalog number ALFL10925.0F
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
2500 mL
Chemical Identifiers
CAS66-25-1
IUPAC Namehexanal
Molecular FormulaC6H12O
InChI KeyJARKCYVAAOWBJS-UHFFFAOYSA-N
SMILESCCCCCC=O
View more
Specifications Specification Sheet
Specification Sheet
Density(20°C) 0.810 to 0.820 g/ml
GC>=95 %
Infrared spectrumConforms
Acid value=<10 mg KOH/g
Appearance (Form)Liquid
View more
Ethyl acetate is widely used as a solvent in cleaning of the circuit board. It is most suited for HPLC instruments and spectrophotometers including the UV-visible spectrophotometer, the IR spectrometer and the mass spectrometer. In addition, it can also be used as a solvent in column chromatography and solvent extraction. It is also used in glues, nail polish removers, decaffeinating tea and coffee and in cigarettes. It is present in confectioneries, perfumes and fruits.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Ethyl acetate is widely used as a solvent in cleaning of the circuit board. It is most suited for HPLC instruments and spectrophotometers including the UV-visible spectrophotometer, the IR spectrometer and the mass spectrometer. In addition, it can also be used as a solvent in column chromatography and solvent extraction. It is also used in glues, nail polish removers, decaffeinating tea and coffee and in cigarettes. It is present in confectioneries, perfumes and fruits.
Solubility
Miscible with ethanol, acetone, diethyl ether and benzene.
Notes
It is flammable and may be moisture sensitive.
Ethyl acetate is widely used as a solvent in cleaning of the circuit board. It is most suited for HPLC instruments and spectrophotometers including the UV-visible spectrophotometer, the IR spectrometer and the mass spectrometer. In addition, it can also be used as a solvent in column chromatography and solvent extraction. It is also used in glues, nail polish removers, decaffeinating tea and coffee and in cigarettes. It is present in confectioneries, perfumes and fruits.
Solubility
Miscible with ethanol, acetone, diethyl ether and benzene.
Notes
It is flammable and may be moisture sensitive.
RUO – Research Use Only
General References:
- Petibon, R.; Harlow, J.; Le, D. B.; Dahn, J. R. The use of ethyl acetate and methyl propanoate in combination with vinylene carbonate as ethylene carbonate-free solvent blends for electrolytes in Li-ion batteries. Electrochim. Acta 2015, 154, 227-234.
- Orozco, D. C., Alarcon, E.; Villa, A. L. Kinetic study of the nopol synthesis by the Prins reaction over tin impregnated MCM-41 catalyst with ethyl acetate as solvent. Fuel 2015, 149, 130-137.