Search Thermo Fisher Scientific
Thermo Scientific Chemicals
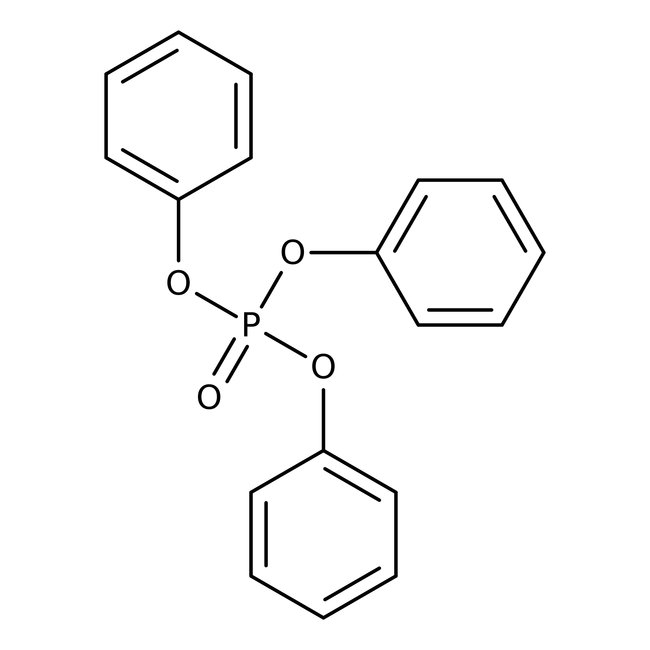
Triphenyl phosphate, 98%, Thermo Scientific Chemicals
CAS: 115-86-6 | C18H15O4P | 326.29 g/mol
Catalog number ALFL08130.30
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
250 g
Chemical Identifiers
CAS83-72-7
IUPAC Name4-hydroxy-1,2-dihydronaphthalene-1,2-dione
Molecular FormulaC10H6O3
InChI KeyWVCHIGAIXREVNS-UHFFFAOYSA-N
SMILESOC1=CC(=O)C(=O)C2=CC=CC=C12
View more
Specifications Specification Sheet
Specification Sheet
Appearance (Form)Crystalline powder
HPLC>=98.5 %
Appearance (Color)Yellow to orange to brown
Infrared spectrumConforms
Triphenyl phosphate is used as an internal standard in the screening and quantification of agrochemical residues in vegetables and fruits. It is used in plastic processing and molding of plastic flow and performance.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Triphenyl phosphate is used as an internal standard in the screening and quantification of agrochemical residues in vegetables and fruits. It is used in plastic processing and molding of plastic flow and performance.
Solubility
Reacts with water.
Notes
Incompatible with oxidizing agents and moisture. Store under dry inert gas. Protect from humidity and water.
Triphenyl phosphate is used as an internal standard in the screening and quantification of agrochemical residues in vegetables and fruits. It is used in plastic processing and molding of plastic flow and performance.
Solubility
Reacts with water.
Notes
Incompatible with oxidizing agents and moisture. Store under dry inert gas. Protect from humidity and water.
RUO – Research Use Only
General References:
- Kristin H Pawlowski and Bernhard Schartel. Flame retardancy mechanisms of triphenyl phosphate, resorcinol bis(diphenyl phosphate) and bisphenol A bis(diphenyl phosphate) in polycarbonate/acrylonitrile-butadiene-styrene blends.Polymer International.2007, 56 1404-1414.
- Kyongho Lee; Jinhwan Kim; Jinyoung Bae; Jaeho Yang; Sanghyun Hong; Hak-Kil Kim. Studies on the thermal stabilization enhancement of ABS; synergistic effect by triphenyl phosphate and epoxy resin mixtures.Polymer.2002, 43 2249-2253.
- Reaction with a secondary alcohol in the presence of a base, e.g. quinoline, gives the diphenyl phosphate ester of the alcohol. This undergoes thermal elimination in sulfolane or NMP to provide the corresponding alkene. The method has clear advantages in terms of yield and toxicity over analogous routes to alkenes, such as the Chugaev xanthate thermolysis or heating the alcohol in HMPA: Synthesis, 1300 (1995). See also Diphenyl phosphorochloridate, A13546.