Search Thermo Fisher Scientific
Thermo Scientific Chemicals
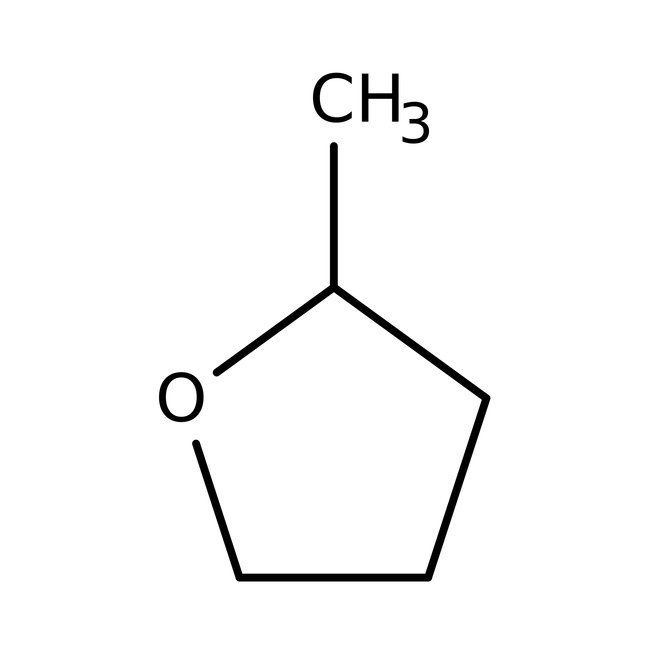
2-Methyltetrahydrofuran, 99%, stab. with ca 150-400ppm BHT, Thermo Scientific Chemicals
CAS: 96-47-9 | C5H10O | 86.134 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFL07356.AP | 500 mL |
Catalog number ALFL07356.AP
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
500 mL
Specifications
Chemical Name or Material2-Methyltetrahydrofuran
Melting Point-136°C
Name Notestabilized with ≈150 to 400ppm BHT
CAS96-47-9
Health Hazard 1H225-H302-H315-H318-H335
View more
2-Methyltetrahydrofuran acts as a solvent in organic synthesis. It is considered as a replacement for terahydrofuran due to its higher reaction temperature and easy separation after reaction. It is also useful in the electrolyte formulation for secondary lithium electrodes and as a component in alternative fuels. Further, it is used as a solvent for spectroscopic studies at -1960C. It also acts as a solvent for Grignard reagent in organometallic reactions. In addition to this, it plays an important role as a motor fuel.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
2-Methyltetrahydrofuran acts as a solvent in organic synthesis. It is considered as a replacement for terahydrofuran due to its higher reaction temperature and easy separation after reaction. It is also useful in the electrolyte formulation for secondary lithium electrodes and as a component in alternative fuels. Further, it is used as a solvent for spectroscopic studies at -1960C. It also acts as a solvent for Grignard reagent in organometallic reactions. In addition to this, it plays an important role as a motor fuel.
Solubility
Miscible with water and most organic solvents.
Notes
Incompatible with oxidizing agents, strong acids and strong bases.
2-Methyltetrahydrofuran acts as a solvent in organic synthesis. It is considered as a replacement for terahydrofuran due to its higher reaction temperature and easy separation after reaction. It is also useful in the electrolyte formulation for secondary lithium electrodes and as a component in alternative fuels. Further, it is used as a solvent for spectroscopic studies at -1960C. It also acts as a solvent for Grignard reagent in organometallic reactions. In addition to this, it plays an important role as a motor fuel.
Solubility
Miscible with water and most organic solvents.
Notes
Incompatible with oxidizing agents, strong acids and strong bases.
RUO – Research Use Only
General References:
- Cryosolvent. Has been proposed as a chlorine-free alternative solvent to dichloromethane in two-phase, including acylation and phase transfer alkylation, reactions. The water-saturated system (ca 39 mL/L) is more polar than the dry solvent, and it forms a useful azeotrope with water (ca 9 : 1), facilitating drying: Synlett, 2353 (2003).
- Phanopoulos, A.; White, A. J. P.; Long, N. J.; Miller, P. W. Catalytic Transformation of Levulinic Acid to 2-Methyltetrahydrofuran Using Ruthenium-N-Triphos Complexes. ACS Catal. 2015, 5 (4), 2500-2512.
- Patankar, S. C.; Yadav, G. D. Cascade Engineered Synthesis of gama-Valerolactone, 1,4-Pentanediol, and 2-Methyltetrahydrofuran from Levulinic Acid Using Pd-Cu/ZrO2 Catalyst in Water as Solvent. ACS Sustainable Chem. Eng. 2015, 3 (11), 2619-2630.