Search Thermo Fisher Scientific
Thermo Scientific Chemicals
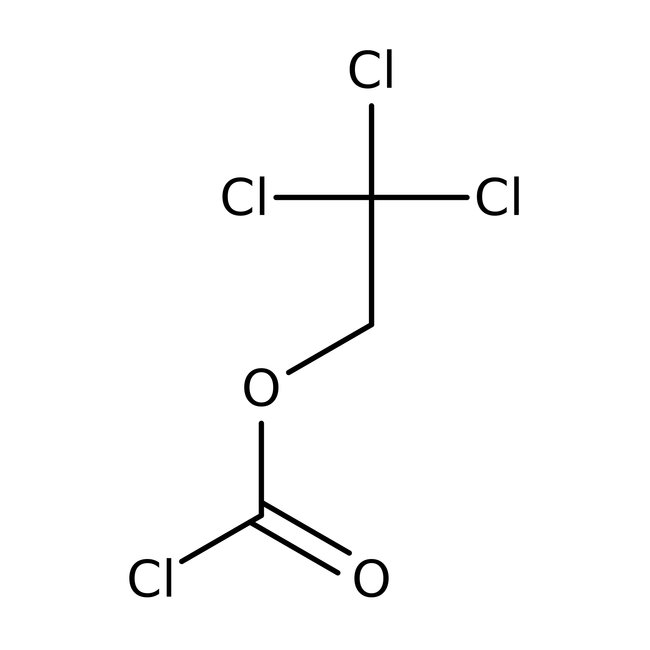
2,2,2-Trichloroethyl chloroformate, 97%, Thermo Scientific Chemicals
CAS: 17341-93-4 | C3H2Cl4O2 | 211.847 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFL06875.22 | 100 g |
Catalog number ALFL06875.22
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
100 g
Chemical Identifiers
CAS13670-99-0
IUPAC Name1-(2,6-difluorophenyl)ethan-1-one
Molecular FormulaC8H6F2O
InChI KeyVGIIILXIQLXVLC-UHFFFAOYSA-N
SMILESCC(=O)C1=C(F)C=CC=C1F
View more
Specifications Specification Sheet
Specification Sheet
FormLiquid
Appearance (Color)Clear colorless to yellow
Assay (GC)≥97.5%
Refractive Index1.4790-1.4830 @ 20?C
2,2,2-Trichloroethyl chloroformate is used as a protecting reagent for aliphatic and aromatic hydroxyl and amino groups. It is used as derivatizing reagent in gas chromatographic/mass spectrometric determination of a large range of amphetamine-related drugs and ephedrines in plasma, urine and hair samples, during N-demethylation of dextromethorphan. It was used as starting reagent during the synthesis of 6-Nor-9,10-dihydrolysergic acid methyl ester.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
2,2,2-Trichloroethyl chloroformate is used as a protecting reagent for aliphatic and aromatic hydroxyl and amino groups. It is used as derivatizing reagent in gas chromatographic/mass spectrometric determination of a large range of amphetamine-related drugs and ephedrines in plasma, urine and hair samples, during N-demethylation of dextromethorphan. It was used as starting reagent during the synthesis of 6-Nor-9,10-dihydrolysergic acid methyl ester.
Solubility
Soluble in Ether, Benzene, Chloroform, Ethanol. Insoluble in water.
Notes
Moisture Sensitive. Desiccate at 4°C. Store under inert gas. Incompatible materials are Strong oxidizing agents.
2,2,2-Trichloroethyl chloroformate is used as a protecting reagent for aliphatic and aromatic hydroxyl and amino groups. It is used as derivatizing reagent in gas chromatographic/mass spectrometric determination of a large range of amphetamine-related drugs and ephedrines in plasma, urine and hair samples, during N-demethylation of dextromethorphan. It was used as starting reagent during the synthesis of 6-Nor-9,10-dihydrolysergic acid methyl ester.
Solubility
Soluble in Ether, Benzene, Chloroform, Ethanol. Insoluble in water.
Notes
Moisture Sensitive. Desiccate at 4°C. Store under inert gas. Incompatible materials are Strong oxidizing agents.
RUO – Research Use Only
General References:
- Thomas A. Montzka; John D. Matiskella; R.A. Partyka. 2,2,2-trichloroethyl chloroformate: A general reagent for demethylation of tertiary methylamines.Tetrahedron Letters.1974,15 1325-1327.
- Giampietro Frison; Luciano Tedeschi; Donata Favretto; Aikebaier Reheman; Santo Davide Ferrara. Gas chromatography/mass spectrometry determination of amphetamine-related drugs and ephedrines in plasma, urine and hair samples after derivatization with 2,2,2-trichloroethyl chloroformate.Rapid Communications in Mass Spectrometry.2005,19 (7), 919-927.
- Reagent for the protection of OH groups as their trichloroethyl (Troc) carbonates, specifically cleaved by reduction with zinc: Tetrahedron Lett., 2555 (1967). For a systematic study of the protection of amines as their Troc-carbamates, see: Synthesis, 268 (1981).
- Preferred to ethyl chloroformate for the N-demethylation of tert-amines, since the resulting carbamate can be cleaved by reductive elimination: Tetrahedron Lett., 1325 (1974); Synth. Commun., 7, 79 (1977).
- Effective reagent for dehydration of primary amides to nitriles: Synth. Commun., 30, 3047 (2000).
- For a brief survey of uses of this reagent in synthesis, see: Synlett, 1473 (2007).