Search Thermo Fisher Scientific
Thermo Scientific Chemicals
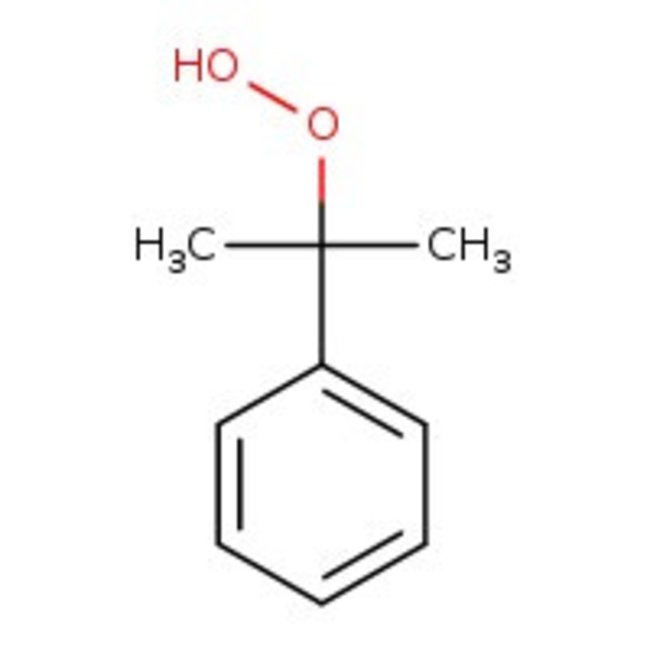
Cumyl hydroperoxide, tech. 80%, Thermo Scientific Chemicals
CAS: 80-15-9 | C9H12O2 | 152.19 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFL06866.36 | 500 g |
Catalog number ALFL06866.36
Price (MYR)
469.00
Quantity:
500 g
Price (MYR)
469.00
Specifications
Chemical Name or MaterialCumene hydroperoxide
CAS80-15-9
Health Hazard 1H226-H242-H302+H312-H304-H314-H331-H335-H350-H373
Health Hazard 2GHS H Statement
H331-H314-H226-H242-H373-H302-H312
Toxic if inhaled.
Causes severe skin burns and eye damage.
Flammable liquid and vapor.
Heating may cause a fire.
May cause damage to organs through prolonged or repeated exposure.
Harmful if swallowed.
Harmful in contact with skin.
H331-H314-H226-H242-H373-H302-H312
Toxic if inhaled.
Causes severe skin burns and eye damage.
Flammable liquid and vapor.
Heating may cause a fire.
May cause damage to organs through prolonged or repeated exposure.
Harmful if swallowed.
Harmful in contact with skin.
Health Hazard 3P201-P202-P210-P220-P233-P234-P235-P240-P241-P242-P243-P260-P264b-P270-P271-P281-P303+P361+P353-P304+P340-P305+P351+P338-P310-P330-P331-P363-P370+P378q-P501c
View more
Cumene hydroperoxide is used in the preparation of polystyrene nanocapsules. It acts as a curing agent for polyester resins and as an oxidizer in organic chemical reactions. It serves as an initiator for radical polymerization especially for acrylate and methacrylate monomers. It also employed as an intermediate in the cumene process for developing phenol and acetone from benzene and propene. Further, it is used as an epoxidation reagent for allylic alcohols and fatty acid esters. In addition to this, it is also used to prepare methylstyrene, acetophenone and cumyl alcohol.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Cumene hydroperoxide is used in the preparation of polystyrene nanocapsules. It acts as a curing agent for polyester resins and as an oxidizer in organic chemical reactions. It serves as an initiator for radical polymerization especially for acrylate and methacrylate monomers. It also employed as an intermediate in the cumene process for developing phenol and acetone from benzene and propene. Further, it is used as an epoxidation reagent for allylic alcohols and fatty acid esters. In addition to this, it is also used to prepare methylstyrene, acetophenone and cumyl alcohol.
Solubility
Miscible with alcohol, acetone, ether, esters, hydrocarbons and chlorinated hydrocarbons. Slightly miscible with water.
Notes
Store in a cool place. Keep the container tightly closed in a dry and well-ventilated place. Incompatible with powdered metals, organic materials, heavy metal salts, metal salts, combustible materials, acids, alkalis, reducing agents, rust, charcoal, amines, copper, lead, cobalt and cobalt oxides.
Cumene hydroperoxide is used in the preparation of polystyrene nanocapsules. It acts as a curing agent for polyester resins and as an oxidizer in organic chemical reactions. It serves as an initiator for radical polymerization especially for acrylate and methacrylate monomers. It also employed as an intermediate in the cumene process for developing phenol and acetone from benzene and propene. Further, it is used as an epoxidation reagent for allylic alcohols and fatty acid esters. In addition to this, it is also used to prepare methylstyrene, acetophenone and cumyl alcohol.
Solubility
Miscible with alcohol, acetone, ether, esters, hydrocarbons and chlorinated hydrocarbons. Slightly miscible with water.
Notes
Store in a cool place. Keep the container tightly closed in a dry and well-ventilated place. Incompatible with powdered metals, organic materials, heavy metal salts, metal salts, combustible materials, acids, alkalis, reducing agents, rust, charcoal, amines, copper, lead, cobalt and cobalt oxides.
RUO – Research Use Only
General References:
- Alternative to tert-Butyl hydroperoxide, A13926, in certain catalytic peroxygenation reactions. For example, has been found to give better enantiomeric excesses in the Sharpless-type asymmetric oxidation of sulfides to sulfoxides in the presence of Ti(O-i-Pr)4 and diethyl L-tartrate: Tetrahedron, 43, 5135 (1987); Org. Synth. Coll., 8, 464 (1993); Synlett, 404 (1996).
- Pardillo-Díaz, R.; Carrascal, L.; Ayala, A.; Nunez-Abades, P. Oxidative stress induced by cumene hydroperoxide evokes changes in neuronal excitability of rat motor cortex neurons. Neuroscience 2015, 289, 85-98.
- Liu, J.; Ni, X.; Hu, Y. Influence of nitridation on the catalytic performance of Ti-MCM-41 for the epoxidation of propene by cumene hydroperoxide. Reac Kinet Mech Cat 2015, 114 (2), 685-695.