Search Thermo Fisher Scientific
Thermo Scientific Chemicals
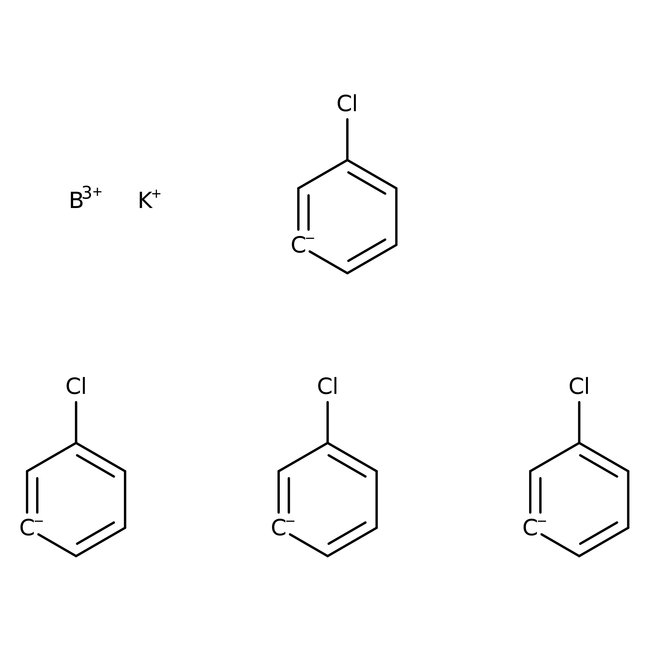
Potassium tetrakis(4-chlorophenyl)borate, 98%, Thermo Scientific Chemicals
CAS: 14680-77-4 | C24H16BCl4K | 496.10 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFL05609.MD | 250 mg |
Catalog number ALFL05609.MD
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
250 mg
Chemical Identifiers
CAS7447-41-8
IUPAC Namelithium(1+) chloride
Molecular FormulaClLi
InChI KeyKWGKDLIKAYFUFQ-UHFFFAOYSA-M
SMILES[Li+].[Cl-]
View more
Specifications Specification Sheet
Specification Sheet
Titration Argentometric>=98.5 %
Appearance (Color)White
Appearance (Form)Crystalline powder or crystals or granules
Loss on drying=<0.5 % (400°C, 4 h)
Insoluble matter=<0.01 % (in acid)
Potassium tetrakis(4-chlorophenyl)borate is used as lipophilic anionic site in constructing fluorescent optode for sodium. It was also used as coating for the piezoelectric crystal in quantifying Potassium using quartz crystal microbalance.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Potassium tetrakis(4-chlorophenyl)borate is used as lipophilic anionic site in constructing fluorescent optode for sodium. It was also used as coating for the piezoelectric crystal in quantifying Potassium using quartz crystal microbalance.
Solubility
Soluble in chloroform.
Notes
Hygroscopic. Store under dry inert gas. Protect from humidity and water. Incompatible with moisture and oxidizing agents.
Potassium tetrakis(4-chlorophenyl)borate is used as lipophilic anionic site in constructing fluorescent optode for sodium. It was also used as coating for the piezoelectric crystal in quantifying Potassium using quartz crystal microbalance.
Solubility
Soluble in chloroform.
Notes
Hygroscopic. Store under dry inert gas. Protect from humidity and water. Incompatible with moisture and oxidizing agents.
RUO – Research Use Only
General References:
- X Yang; K Wang; D Xiao; C Guo; Y Xu. Development of a fluorescent optode membrane for sodium ion based on the calix[4]arene and tetraphenylporphine. Talanta. 2000, 52, (6), 1033-1039.
- M Teresa; S R Gomes; K S Tavares; J A Oliveira. The quantification of potassium using a quartz crystal microbalance. Analyst (Cambridge UK). 2000, 125, (11), 1983-1986.
- Tetraphenylborates cross couple with aryl halides in aqueous solutions with Pd(OAc) 2 catalyst: Dokl. Chem., 315, 354 (1990). See also Sodium tetraphenyl borate, A10909.