Search Thermo Fisher Scientific
Thermo Scientific Chemicals
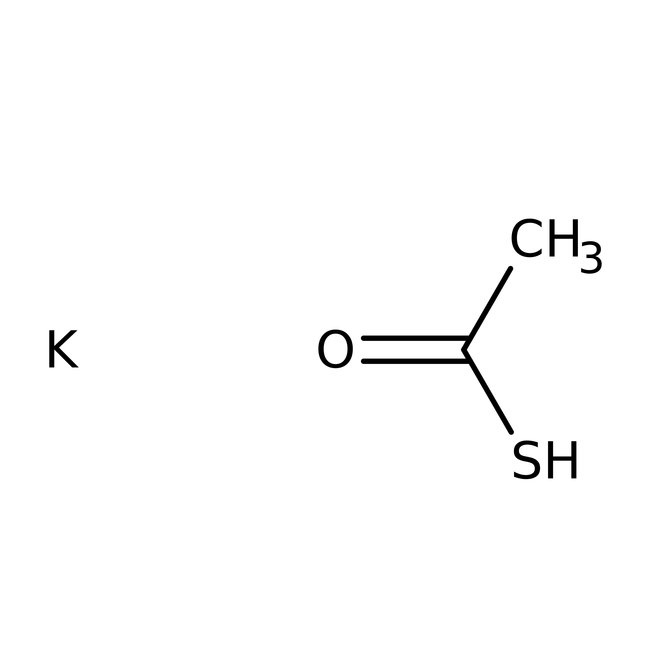
Potassium thioacetate, 98%, Thermo Scientific Chemicals
CAS: 10387-40-3 | C2H4KOS | 115.211 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFL05405.14 | 25 g |
Catalog number ALFL05405.14
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
25 g
Specifications
Chemical Name or MaterialPotassium thioacetate
CAS10387-40-3
Health Hazard 1H315-H319-H335
Health Hazard 2GHS H Statement
H315-H319-H335
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
H315-H319-H335
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
Health Hazard 3P261-P264b-P271-P280-P302+P352-P304+P340-P305+P351+P338-P312-P332+P313-P362-P501c
View more
Potassium thioacetate is used for palladium mediated coupling with aryl halides and triflates leading to S-arylthioacetates and derivatives. It is also used as a reagent in the conversion of halides to thiols.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Potassium thioacetate is used for palladium mediated coupling with aryl halides and triflates leading to S-arylthioacetates and derivatives. It is also used as a reagent in the conversion of halides to thiols.
Solubility
Soluble in water, methanol and some polar solvents. Insoluble in alkanes, ethers and most nonpolar solvents.
Notes
Air sensitive. Hygroscopic. Keep the container tightly closed in a dry and well-ventilated place. Incompatible with strong acids.
Potassium thioacetate is used for palladium mediated coupling with aryl halides and triflates leading to S-arylthioacetates and derivatives. It is also used as a reagent in the conversion of halides to thiols.
Solubility
Soluble in water, methanol and some polar solvents. Insoluble in alkanes, ethers and most nonpolar solvents.
Notes
Air sensitive. Hygroscopic. Keep the container tightly closed in a dry and well-ventilated place. Incompatible with strong acids.
RUO – Research Use Only
General References:
- Reagent for the conversion of halides and sulfonates to thiols via the thiol acetates, thus avoiding the problem of sulfide formation: J. Chem. Soc., 579 (1950).
- Ortega, P.; Gómez, R.; de la Mata, F. J. Thiol ended carbosilane dendrimers. A multivalent platform for the binding of molecules of biological interest. Tetrahedron Lett. 2015, 56 (38), 5299-5302.
- Zhao, J.; Wang, C.; Zhao, P.; Wen, X.; Lin, C. Bioreducible dextran-polyethylenimine conjugates regulate transgene expression distribution in vivo. J. Mater. Chem. B 2015, 3 (8), 1529-1536.