Search Thermo Fisher Scientific
Thermo Scientific Chemicals
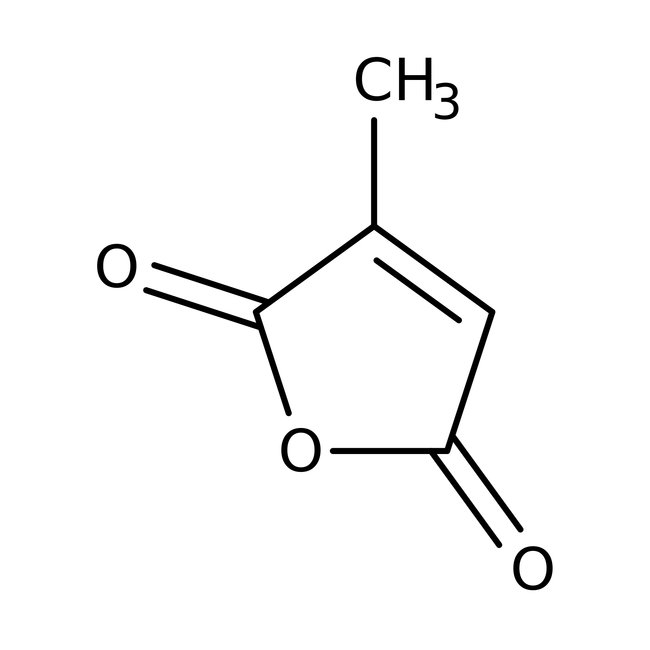
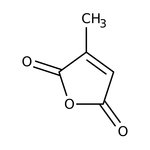
Citraconic anhydride, 98%, Thermo Scientific Chemicals
CAS: 616-02-4 | C5H4O3 | 112.084 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFL05238.14 | 25 g |
Catalog number ALFL05238.14
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
25 g
Chemical Identifiers
CAS56-24-6
IUPAC Nametrimethylstannanol
Molecular FormulaC3H10OSn
InChI KeyOJZNYUDKNVNEMV-UHFFFAOYSA-M
SMILESC[Sn](C)(C)O
View more
Specifications Specification Sheet
Specification Sheet
FormSolid
Appearance (Color)White to off-white
Assay from Suppliers CofA≥97.5%
Citraconic anhydride is used as a monomer for specialty unsaturated polyester resins. It is used to prepare bicyclic pyrrolidines, maleimides and co- and terpolymers. It is also used for the protection of N-terminal amino acids. Further, it is involved in the preparation of clothing ping acid and its amide. In addition to this, it is selectively used for the isolation of histidyl peptides.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Citraconic anhydride is used as a monomer for specialty unsaturated polyester resins. It is used to prepare bicyclic pyrrolidines, maleimides and co- and terpolymers. It is also used for the protection of N-terminal amino acids. Further, it is involved in the preparation of clothing ping acid and its amide. In addition to this, it is selectively used for the isolation of histidyl peptides.
Solubility
Miscible with water, alcohol, acetone and ether.
Notes
Moisture sensitive. Incompatible with bases, oxidizing agents and reducing agents.
Citraconic anhydride is used as a monomer for specialty unsaturated polyester resins. It is used to prepare bicyclic pyrrolidines, maleimides and co- and terpolymers. It is also used for the protection of N-terminal amino acids. Further, it is involved in the preparation of clothing ping acid and its amide. In addition to this, it is selectively used for the isolation of histidyl peptides.
Solubility
Miscible with water, alcohol, acetone and ether.
Notes
Moisture sensitive. Incompatible with bases, oxidizing agents and reducing agents.
RUO – Research Use Only
General References:
- Reagent for the protection of amino groups: Biochem. J., 109, 312 (1968).
- Wang, L.; Wang, H.; Li, Y.; Tang, P. Total Synthesis of Schilancitrilactones B and C. Angew. Chem. Int. Ed. 2015, 54 (19), 5732-5735.
- Eddy, N. A.; Fenteany, G. Model studies directed to the synthesis of cucurbitacin I C/D rings. Tetrahedron Lett. 2015, 56 (36), 5079-5081.