Search Thermo Fisher Scientific
Thermo Scientific Chemicals
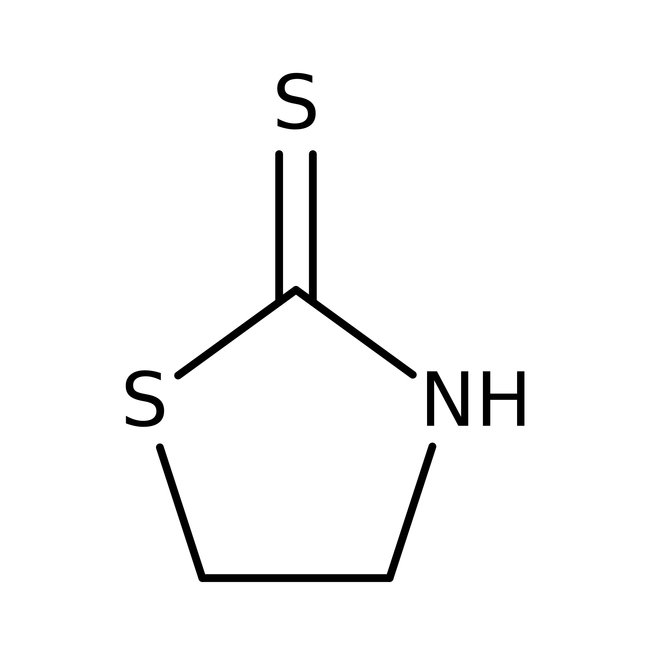
2-Mercaptothiazoline, 98+%, Thermo Scientific Chemicals
CAS: 96-53-7 | C3H5NS2 | 119.2 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFL04924.22 | 100 g |
Catalog number ALFL04924.22
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
100 g
Chemical Identifiers
CAS995-45-9
IUPAC Nametributyl(chloro)silane
Molecular FormulaC12H27ClSi
InChI KeyJSQJUDVTRRCSRU-UHFFFAOYSA-N
SMILESCCCC[Si](Cl)(CCCC)CCCC
View more
Specifications Specification Sheet
Specification Sheet
Assay (GC)≥96.0%
Identification (FTIR)Conforms
Refractive Index1.4445-1.4495 @ 20?C
FormLiquid or viscous liquid
Appearance (Color)Clear colorless to pale yellow
2-Mercaptothiazoline, is used in the chain-lengthening of alkyl halides by trans-iodopropenylenation. 2-Mercaptothiazoline is an antithyroid agent that strongly reduces thyroid hormone levels. 2-Mercaptothiazoline is also used as an intermediate in the preparation of biologically active compounds. It is also used as a tool for highly selective chiral synthesis of penam- and carbapenam-type β-lactam antibiotics.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
2-Mercaptothiazoline, is used in the chain-lengthening of alkyl halides by trans-iodopropenylenation. 2-Mercaptothiazoline is an antithyroid agent that strongly reduces thyroid hormone levels. 2-Mercaptothiazoline is also used as an intermediate in the preparation of biologically active compounds. It is also used as a tool for highly selective chiral synthesis of penam- and carbapenam-type β-lactam antibiotics.
Solubility
Soluble in chloroform, methanol. Slightly soluble in water.
Notes
Keep container tightly closed in a dry and well-ventilated place. Keep away from oxidizing agents.
2-Mercaptothiazoline, is used in the chain-lengthening of alkyl halides by trans-iodopropenylenation. 2-Mercaptothiazoline is an antithyroid agent that strongly reduces thyroid hormone levels. 2-Mercaptothiazoline is also used as an intermediate in the preparation of biologically active compounds. It is also used as a tool for highly selective chiral synthesis of penam- and carbapenam-type β-lactam antibiotics.
Solubility
Soluble in chloroform, methanol. Slightly soluble in water.
Notes
Keep container tightly closed in a dry and well-ventilated place. Keep away from oxidizing agents.
RUO – Research Use Only
General References:
- Lei Tao et. al. Synthesis and bioactivity of poly(HPMA)-lysozyme conjugates: the use of novel thiazolidine-2-thione coupling chemistry. Organic & Biomolecular Chemistry. 2009, 7,(17), 3481-3485 .
- Newton L Dias Filho et. al. Preparation of a clay-modified carbon paste electrode based on 2-thiazoline-2-thiol-hexadecylammonium sorption for the sensitive determination of mercury. Analytical Sciences. 2005, 21,(11), 1309-1316 .
- Used in the chain-lengthening of alkyl halides by trans-iodopropenylenation: Org. Synth. Coll., 6, 704 (1988):
- Reaction with ɑ-bromo esters gives the S-alkoxycarbonylmethyl derivatives, which can be C-alkylated by alkyl halides, using NaH as base. Sulfur-free products can be obtained by reduction (Zn/AcOH) to give the substituted acetic acid, or by reaction with iodidomethane to give the corresponding ɑ-iodo ester: Tetrahedron Lett., 2677 (1977).