Search Thermo Fisher Scientific
Thermo Scientific Chemicals
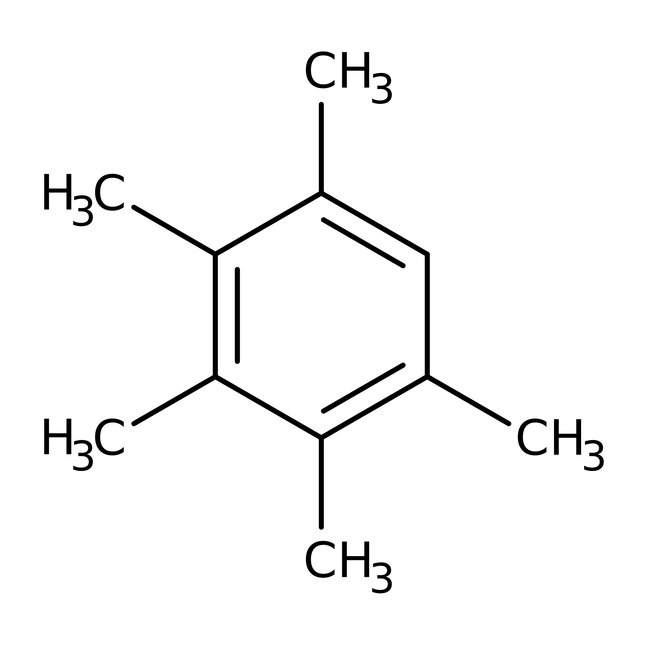
Pentamethylbenzene, 99%, Thermo Scientific Chemicals
CAS: 700-12-9 | C11H16 | 148.249 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFL04163.22 | 100 g |
Catalog number ALFL04163.22
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
100 g
Chemical Identifiers
CAS1400-62-0
IUPAC Name4,12-bis[(2,4-dihydroxy-6-methylphenyl)amino]-3,13-dimethyl-8-oxatricyclo[7.4.0.0²,⁷]trideca-1,3,6,9,12-pentaene-5,11-dione
Molecular FormulaC28H24N2O7
InChI KeyVPEASJIRGSVXBF-UHFFFAOYSA-N
SMILESCC1=CC(O)=CC(O)=C1NC1=C(C)C2=C3C(OC2=CC1=O)=CC(=O)C(NC1=C(C)C=C(O)C=C1O)=C3C
View more
Specifications Specification Sheet
Specification Sheet
Appearance (Form)Powder
Appearance (Color)Brown to dark purple to black
Infrared spectrumConforms
UV Lambda max575 to 583 nm
Absorption Ratio P-15/P+150.98 to 1.18
View more
Pentamethylbenzene is used to prepare a mixture of nitropentamethylbenzene and 2,3,4,5-tetramethylbenzyl nitrate1. It was also used to prepare propene.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Pentamethylbenzene is used to prepare a mixture of nitropentamethylbenzene and 2,3,4,5-tetramethylbenzyl nitrate1. It was also used to prepare propene.
Notes
Incompatible with oxidizing agents. Store in cool place. Keep container tightly closed in a dry and well-ventilated place.
Pentamethylbenzene is used to prepare a mixture of nitropentamethylbenzene and 2,3,4,5-tetramethylbenzyl nitrate1. It was also used to prepare propene.
Notes
Incompatible with oxidizing agents. Store in cool place. Keep container tightly closed in a dry and well-ventilated place.
RUO – Research Use Only
General References:
- Yingxin Sun; Sheng Han. Mechanistic investigation of methanol to propene conversion catalyzed by H-beta zeolite: a two-layer ONIOM study.Journal of Molecular Modeling.2013, 19 (12), 5407-5422 .
- S Bhattacharya; M Banerjee; A K Mukherjee. Study of the formation equilibria of electron donor-acceptor complexes between [60]fullerene and methylbenzenes by absorption spectrometric method.Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy.2001, 57 (7), 1463-1470 .
- Can be used as a carbocation scavenger in the acid-promoted deblocking of peptides, e.g., t-butyl cations in the cleavage of N-Boc groups with TFA: Coll. Czech. Chem. Commun., 55, 1792 (1990). Similarly, has been used as an additive in the cleavage of O-benzyl and N-Cbz (Z) groups: Chem. Pharm. Bull., 35, 3438 (1987). See also Appendix 6.