Search Thermo Fisher Scientific
Thermo Scientific Chemicals
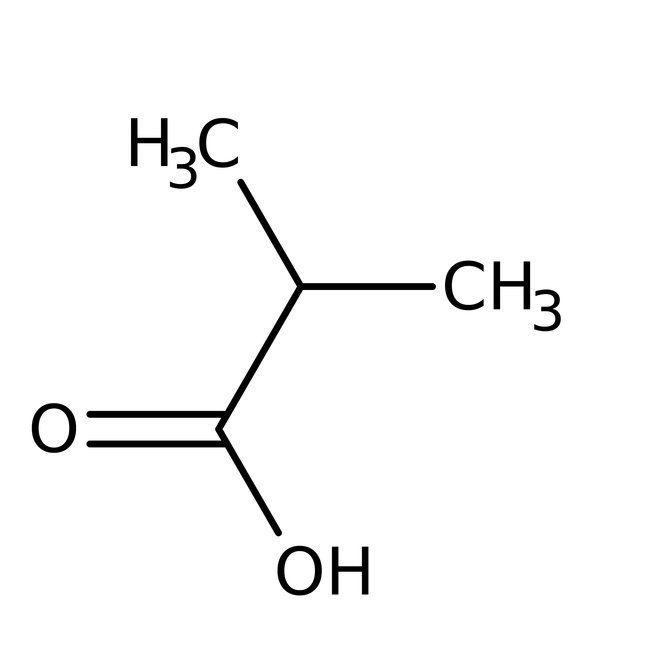
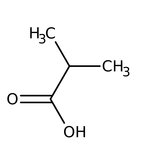
Isobutyric acid, 99%, Thermo Scientific Chemicals
CAS: 79-31-2 | C4H8O2 | 88.11 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFL04038.AE | 100 mL |
Catalog number ALFL04038.AE
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
100 mL
Chemical Identifiers
CAS98-85-1
IUPAC Name1-phenylethan-1-ol
Molecular FormulaC8H10O
InChI KeyWAPNOHKVXSQRPX-UHFFFAOYNA-N
SMILESCC(O)C1=CC=CC=C1
View more
Specifications Specification Sheet
Specification Sheet
FormLiquid
Identification (FTIR)Conforms
Appearance (Color)Clear colorless
Refractive Index1.5250-1.5300 @ 20?C
Assay (GC)≥96.0%
Isobutyric acid is used to prepare esters for flavors and perfumes. It is also used as a disinfecting agent, preservative and tanning agent. It finds applications in textile, varnish and the leather industry. Further, it is used as a lactation stimulant in dairy cattle. In addition to this, it is used in the preparation of tetramethylsuccinic acid and diisopropyl ketone.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Isobutyric acid is used to prepare esters for flavors and perfumes. It is also used as a disinfecting agent, preservative and tanning agent. It finds applications in textile, varnish and the leather industry. Further, it is used as a lactation stimulant in dairy cattle. In addition to this, it is used in the preparation of tetramethylsuccinic acid and diisopropyl ketone.
Solubility
Miscible with water, alcohol, chloroform and ether. Slightly miscible with carbon tetrachloride.
Notes
Incompatible with strong oxidizing agents.
Isobutyric acid is used to prepare esters for flavors and perfumes. It is also used as a disinfecting agent, preservative and tanning agent. It finds applications in textile, varnish and the leather industry. Further, it is used as a lactation stimulant in dairy cattle. In addition to this, it is used in the preparation of tetramethylsuccinic acid and diisopropyl ketone.
Solubility
Miscible with water, alcohol, chloroform and ether. Slightly miscible with carbon tetrachloride.
Notes
Incompatible with strong oxidizing agents.
RUO – Research Use Only
General References:
- Tashiro, Y.; Rodriguez, G. M.; Atsumi, S. 2-Keto acids based biosynthesis pathways for renewable fuels and chemicals. J. Ind. Microbiol. Biotechnol. 2015, 42 (3), 361-373.
- Luo, L.; Liu, D.; Li, L.; Chen, Y. Phase equilibria of (water + propionic acid or butyric acid + 2-methoxy-2-methylpropane) ternary systems at 298.2 K and 323.2 K. Fluid Phase Equilib. 2015, 403, 30-35.