Search Thermo Fisher Scientific
Thermo Scientific Chemicals
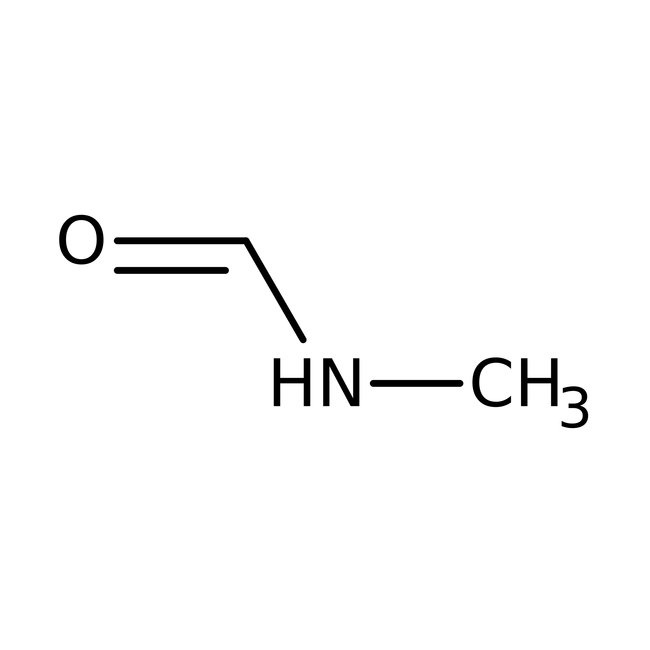
N-Methylformamide, 99%, Thermo Scientific Chemicals
CAS: 123-39-7 | C2H5NO | 59.068 g/mol
Catalog number ALFL03908.0B
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
1000 g
Chemical Identifiers
CAS4965-09-7
IUPAC Name1-methyl-1,2,3,4-tetrahydroisoquinoline
Molecular FormulaC10H13N
InChI KeyQPILYVQSKNWRDD-UHFFFAOYNA-N
SMILESCC1NCCC2=CC=CC=C12
View more
Specifications Specification Sheet
Specification Sheet
Assay (GC)>94.0%
N-Methylformamide is used to prepare methyl isocyanate. It is used as an extraction solvent for aromatic hydrocarbons and acts as a ligand in coordination chemistry. It is employed as a solvent in oil refineries and in aluminum electrolytic capacitors. Further, it is used as a reagent in organic synthesis. In addition to this, it is used as an investigational anticancer drug and an intermediate in the production of some pharmaceutical compounds.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
N-Methylformamide is used to prepare methyl isocyanate. It is used as an extraction solvent for aromatic hydrocarbons and acts as a ligand in coordination chemistry. It is employed as a solvent in oil refineries and in aluminum electrolytic capacitors. Further, it is used as a reagent in organic synthesis. In addition to this, it is used as an investigational anticancer drug and an intermediate in the production of some pharmaceutical compounds.
Solubility
Miscible with water, acetone and ethanol. Immiscible with ether.
Notes
Incompatible with strong oxidizing agents, acids, bases and acid chlorides.
N-Methylformamide is used to prepare methyl isocyanate. It is used as an extraction solvent for aromatic hydrocarbons and acts as a ligand in coordination chemistry. It is employed as a solvent in oil refineries and in aluminum electrolytic capacitors. Further, it is used as a reagent in organic synthesis. In addition to this, it is used as an investigational anticancer drug and an intermediate in the production of some pharmaceutical compounds.
Solubility
Miscible with water, acetone and ethanol. Immiscible with ether.
Notes
Incompatible with strong oxidizing agents, acids, bases and acid chlorides.
RUO – Research Use Only
General References:
- Reagent for replacement of an activated chlorine in an aromatic or heteroaromatic ring by the methylamino group: Synthesis, 39 (1980).
- Precursor of methyl isocyanide (highly toxic!), by dehydration with tosyl chloride in pyridine: Org. Synth. Coll., 5, 772 (1973).
- Emets, V. V.; Damaskin, B. B. Two-dimensional pressure of chemisorbed dimethylformamide and N-methylformamide molecules at Ga-, (In-Ga)-, and (Tl-Ga)-electrodes. Russ. J. Electrochem. 2015, 51 (8), 789-795.
- Bunkan, A. J. C.; Hetzler, J.; Mikoviny, T.; Wisthaler, A.; Nielsen, C. J.; Olzmann, M. The reactions of N-methylformamide and N,N-dimethylformamide with OH and their photo-oxidation under atmospheric conditions: experimental and theoretical studies. Phys. Chem. Chem. Phys. 2015, 17, 7046-7059.