Search Thermo Fisher Scientific
Thermo Scientific Chemicals
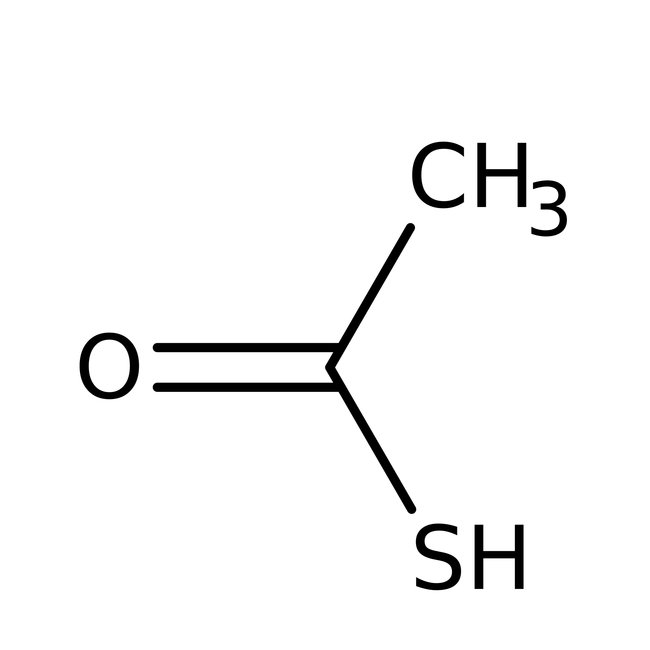
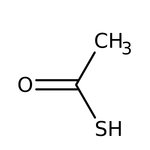
Thioacetic acid, 97%, Thermo Scientific Chemicals
CAS: 507-09-5 | C2H4OS | 76.11 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFL03305.14 | 25 g |
Catalog number ALFL03305.14
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
25 g
Chemical Identifiers
CAS108-94-1
IUPAC Namecyclohexanone
Molecular FormulaC6H10O
InChI KeyJHIVVAPYMSGYDF-UHFFFAOYSA-N
SMILESO=C1CCCCC1
View more
Specifications Specification Sheet
Specification Sheet
Appearance (Form)Clear liquid
Color scale=<20 APHA
Infrared spectrumConforms
GC>=99.75 %
Water=<0.2 % (K.F.)
View more
Thioacetic acid is a reagent for introduction of the thiol group into organic molecules. It is used as flavor & Fragrance Intermediates. It is used to manufacture captopril(antihypertension) and spironolac-tone (diuretic).
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Thioacetic acid is a reagent for introduction of the thiol group into organic molecules. It is used as flavor & Fragrance Intermediates. It is used to manufacture captopril(antihypertension) and spironolac-tone (diuretic).
Solubility
Soluble in water. (27g/L) at 16°C.
Notes
Protect from heat. Store away from oxidizing agents. Incompatible with strong bases, strong oxidizing agents, metals.
Thioacetic acid is a reagent for introduction of the thiol group into organic molecules. It is used as flavor & Fragrance Intermediates. It is used to manufacture captopril(antihypertension) and spironolac-tone (diuretic).
Solubility
Soluble in water. (27g/L) at 16°C.
Notes
Protect from heat. Store away from oxidizing agents. Incompatible with strong bases, strong oxidizing agents, metals.
RUO – Research Use Only
General References:
- Wen Yang; Da-Ming Du. Squaramide-catalysed enantio- and diastereoselective sulfa-Michael addition of thioacetic acid to α,β-disubstituted nitroalkenes.Organic & Biomolecular Chemistry.2012, 10 (34), 6876-6884.
- Libo Hu; Hui Zhu; Da-Ming Du; Jiaxi Xu. Efficient synthesis of taurine and structurally diverse substituted taurines from aziridines.Journal of Organic Chemistry.2007, 72 (12), 4543-4546.
- Used in several different ways for the introduction of the thiol group: Radical addition to unactivated alkenes: J. Chem. Soc., 2123 (1951). Conjugate (Michael) addition to activated double bonds: J. Am. Chem. Soc., 77, 5144 (1955); 81, 1224 (1959). Nucleophilic displacement of halide, tosylate or acetate: J. Am. Chem. Soc., 73, 2659 (1951); Carbohydr. Res., 17, 457 (1971). Alcohols can be converted to thiol esters under Mitsunobu conditions, followed by LAH reduction to the thiol: Tetrahedron Lett., 3119 (1981).