Search Thermo Fisher Scientific
Thermo Scientific Chemicals
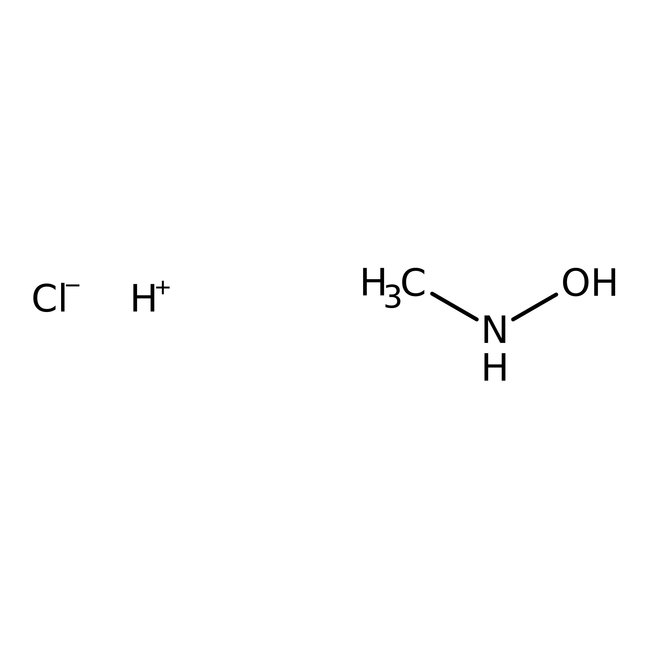
N-Methylhydroxylamine hydrochloride, 98%, Thermo Scientific Chemicals
CAS: 4229-44-1 | CH6ClNO | 83.52 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFL02202.06 | 5 g |
Catalog number ALFL02202.06
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
5 g
Chemical Identifiers
CAS7440-06-4
IUPAC Nameplatinum
Molecular FormulaPt
InChI KeyBASFCYQUMIYNBI-UHFFFAOYSA-N
SMILES[Pt]
View more
N-Methylhydroxylamine hydrochloride is used as an inorganic catalyst used in the transamidation of primary amides with amines. It is employed in constructing hydroxamates, important functional groups for the complexation of iron.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
N-Methylhydroxylamine hydrochloride is used as an inorganic catalyst used in the transamidation of primary amides with amines. It is employed in constructing hydroxamates, important functional groups for the complexation of iron.
Solubility
Soluble in water.
Notes
Hygroscopic. Store under dry inert gas. Protect from humidity and air.
N-Methylhydroxylamine hydrochloride is used as an inorganic catalyst used in the transamidation of primary amides with amines. It is employed in constructing hydroxamates, important functional groups for the complexation of iron.
Solubility
Soluble in water.
Notes
Hygroscopic. Store under dry inert gas. Protect from humidity and air.
RUO – Research Use Only
General References:
- A. Gibson and S. Mirzazadeh. N-methylhydroxylamine inhibits and M&B 22948 potentiates relaxations of the mouse anococcygeus to non-adrenergic, non-cholinergic field stimulation and to nitrovasodilator drugs.British Journal of Pharmacology.1989, 96 637-644.
- J. R. Ochoa Gomez. Electrosynthesis of N-methylhydroxylamine.Journal of Applied Electrochemistry.1991, 21 331-334.
- Reacts with aldehydes and ketones to give nitrones, which undergo 1,3-dipolar addition reactions with alkenes. Cycloaddition to trimethylvinylsilane has been used in a 2-carbon extension of aldehydes to ɑß-unsaturated aldehydes: J. Org. Chem., 49, 3421 (1984):
- Intramolecular cycloaddition has been used as a route to bicyclic systems, e.g. from citronellal: Org. Synth. Coll., 6, 670 (1988).
- Reviews: 1,3-Dipolar cycloadditions of nitrones: Synthesis, 205 (1975). The [3+2] nitrone-olefin cycloaddition reaction: Org. React., 36, 1 (1988). Synthetic applications of nitrones: Org. Prep. Proced. Int., 17, 25 (1985).
- For conversion to, and reactions of, the N,O-bis(TMS) derivative, see: J. Chem. Soc., Perkin 1, 1823 (1989).