Search Thermo Fisher Scientific
Thermo Scientific Chemicals
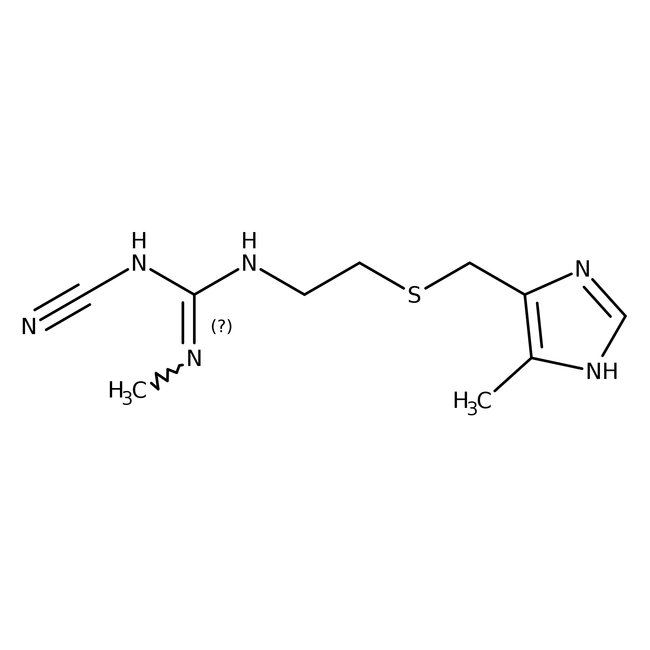
Cimetidine, 98+%, Thermo Scientific Chemicals
H2 histamine receptor antagonist
| Catalog Number | Quantity |
|---|---|
| ALFJ62825.06 | 5 g |
Catalog number ALFJ62825.06
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
5 g
Chemical Identifiers
CAS540-84-1
IUPAC Name2,2,4-trimethylpentane
Molecular FormulaC8H18
InChI KeyNHTMVDHEPJAVLT-UHFFFAOYSA-N
SMILESCC(C)CC(C)(C)C
View more
Specifications Specification Sheet
Specification Sheet
Color scale=<10 APHA
GC/ECD (as lindane)=<5 ng/l
GC>=99.5 %
Acidity (CH3COOH)=<0.003 %
Appearance (Form)Clear liquid
View more
Widely used H2 histamine antagonist which has more recently been described as an inverse agonist. Also a potent I1 imidazoline binding site ligand. It displays antitumor and immunomodulatory activity. Cimetidine competitively inhibits histamine binding to histamine H2 receptors, and suppresses the growth of several tumors, including gastrointestinal cancer. This compound is an anti-angiogenic agent and is an activator of Nischarin. Competitive histamine H2-receptor antagonist which inhibits gastric acid secretion and reduces pepsin output
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Solubility
Soluble to 50 mM in water and to 100 mM in DMSO, solubility in HCl (almost transparency).
Notes
Store away from heat and oxidizing agents. Keep the container tightly closed and place it in a cool, dry and well ventilated condition.
Soluble to 50 mM in water and to 100 mM in DMSO, solubility in HCl (almost transparency).
Notes
Store away from heat and oxidizing agents. Keep the container tightly closed and place it in a cool, dry and well ventilated condition.
RUO – Research Use Only
General References:
- Yasui-Furukori N, et al. Different effects of three transporting inhibitors, verapamil, cimetidine, and probenecid, on fexofenadine pharmacokinetics.Clinical pharmacology and therapeutics.,2005,77(1), 17-23.
- Yoshizawa F, et al. Time course of leucine-induced 4E-BP1 and S6K1 phosphorylation in the liver and skeletal muscle of rats.Journal of clinical pharmacology.,1981,21(2), 87-91.