Search Thermo Fisher Scientific
Thermo Scientific Chemicals
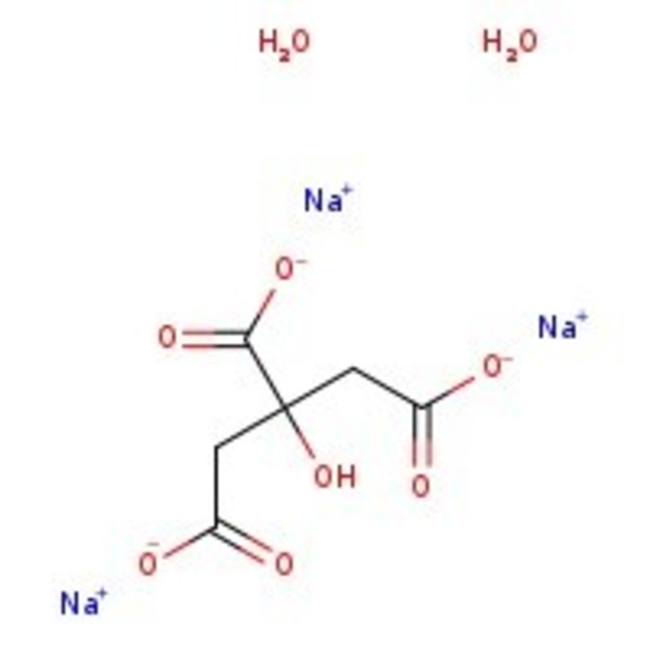
Citrate, 0.5M buffer soln., pH 4.0
CAS: 6132-04-3294.10 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFJ61249.AP | 500 mL |
Catalog number ALFJ61249.AP
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
500 mL
Chemical Identifiers
CAS9002-92-0
MDL NumberMFCD00043063
Specifications Specification Sheet
Specification Sheet
Appearance (Color)Clear colorless
Appearance (Form)Jelly substance
Assay29 to 31 %
Citrate, 0.5M buffer soln., pH 4.0 may be used for antigen retrieval of tissue samples. The citrate solution is designed to break protein cross-links; thus, unmasking antigens and epitopes in formalin-fixed and paraffin embedded tissue sections, resulting in enhancing staining intensity of antibodies. Citrate has anticoagulant activity as a calcium chelator.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Citrate, 0.5M buffer soln., pH 4.0 may be used for antigen retrieval of tissue samples. The citrate solution is designed to break protein cross-links; thus, unmasking antigens and epitopes in formalin-fixed and paraffin embedded tissue sections, resulting in enhancing staining intensity of antibodies. Citrate has anticoagulant activity as a calcium chelator.
Solubility
It is soluble in water.
Notes
Store at room temperature.
Citrate, 0.5M buffer soln., pH 4.0 may be used for antigen retrieval of tissue samples. The citrate solution is designed to break protein cross-links; thus, unmasking antigens and epitopes in formalin-fixed and paraffin embedded tissue sections, resulting in enhancing staining intensity of antibodies. Citrate has anticoagulant activity as a calcium chelator.
Solubility
It is soluble in water.
Notes
Store at room temperature.
RUO – Research Use Only
General References:
- JM Chirgwin.; AE Przybyla.; RJ MacDonald. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979, 18,(24), 5294-5299.
- PJ Elving.; JM Markowitz.; I Rosenthal. Preparation of buffer systems of constant ionic strength. Analytical Chemistry. 1956, 28,(7), 1179-1180.
Contents & Storage
Keep cold