Search Thermo Fisher Scientific
Thermo Scientific Chemicals
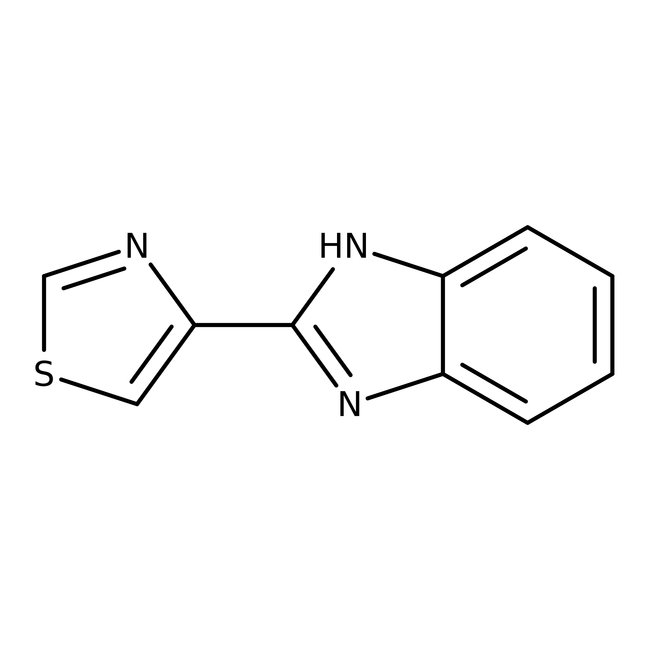
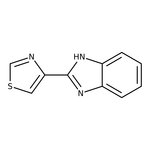
Thiabendazole, 98+%, Thermo Scientific Chemicals
Fungicide and antihelminthic
Catalog number ALFJ60009.22
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
100 g
Chemical Identifiers
CAS1465-25-4
IUPAC Namedihydrogen N1-(naphthalen-1-yl)ethane-1,2-diamine dichloride
Molecular FormulaC12H16Cl2N2
InChI KeyMZNYWPRCVDMOJG-UHFFFAOYSA-N
SMILES[H+].[H+].[Cl-].[Cl-].NCCNC1=C2C=CC=CC2=CC=C1
View more
Specifications Specification Sheet
Specification Sheet
Appearance (Color)White to cream or pale grey
Formpowder
Assay (Titration ex Chloride)> 95.0% (dry wt. basis)
Water Content (Karl Fischer Titration)< 5.0%
Thiabendazole, a metal chelator and helminth-specific fumarate reductase inhibitor, is used primarily as an anthelmintic to control infections by helminth organisms. It is also used to control mold and fungal growth in plants. Also used to treat parasitic infections in humans. It is used clinically to treat threadworm, cutaneous larva migrans, visceral larva migrans, and trichinosis.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Solubility
Soluble in methanol and dimethyl sulfoxide.
Notes
Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Store away from oxidizing agent.
Soluble in methanol and dimethyl sulfoxide.
Notes
Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Store away from oxidizing agent.
RUO – Research Use Only
General References:
- Roslyn S Thelingwani; Simbarashe P Zvada; Hugues Dolgos; Anna-Lena B Ungell; Collen M Masimirembwa. In vitro and in silico identification and characterization of thiabendazole as a mechanism-based inhibitor of CYP1A2 and simulation of possible pharmacokinetic drug-drug interactions. Drug Metabolism and Disposition, 2009, 37(6), 1286-1294.
- Craig Knox et. al. DrugBank 3.0: a comprehensive resource for 'omics' research on drugs. Nucleic Acids Research, 2012, 39(3), D1035-D1041.