Search Thermo Fisher Scientific
Thermo Scientific Chemicals
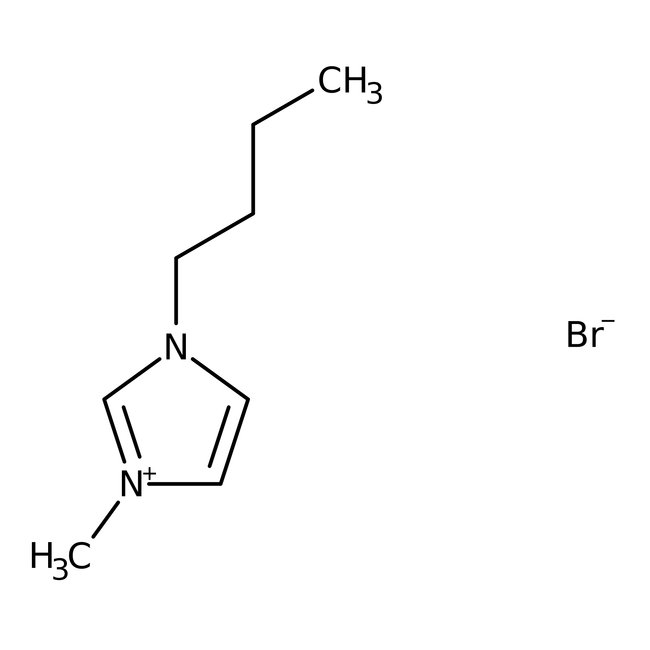
1-n-Butyl-3-methylimidazolium bromide, 99%, Thermo Scientific Chemicals
CAS: 85100-77-2 | C8H15BrN2 | 219.126 g/mol
Catalog number ALFH27201.18
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
50 g
Chemical Identifiers
CAS7440-44-0
IUPAC Namecarbon
Molecular FormulaC
InChI KeyOKTJSMMVPCPJKN-UHFFFAOYSA-N
SMILES[C]
View more
It is an ionic liquid used in a Heck reaction. It is also used in assessing the factors responsible for ionic liquid toxicity to aquatic organisms via quantitative structure-property relationship modeling
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
It is an ionic liquid used in a Heck reaction. It is also used in assessing the factors responsible for ionic liquid toxicity to aquatic organisms via quantitative structure-property relationship modeling
Solubility
Miscible in water, methanol and dichloromethane. Immiscible in acetone, toluene, ethyl acetate and diethyl ether.
Notes
Hygroscopic. Store away from oxidizing agents. Keep the container tightly closed and place it in a cool, dry and well ventilated condition.
It is an ionic liquid used in a Heck reaction. It is also used in assessing the factors responsible for ionic liquid toxicity to aquatic organisms via quantitative structure-property relationship modeling
Solubility
Miscible in water, methanol and dichloromethane. Immiscible in acetone, toluene, ethyl acetate and diethyl ether.
Notes
Hygroscopic. Store away from oxidizing agents. Keep the container tightly closed and place it in a cool, dry and well ventilated condition.
RUO – Research Use Only
General References:
- M. Teresa Garcia, et al. Biodegradable ionic liquids Part II. Effect of the anion and toxicology.Green Chem.,2005,7(1), 9-14.
- Miriam Kohagen, et al. How Hydrogen Bonds Influence the Mobility of Imidazolium-Based Ionic Liquids. A Combined Theoretical and Experimental Study of 1-n-Butyl-3-methylimidazolium Bromide.J. Phys. Chem. B.,2011,115(51), 15280-15288.