Search Thermo Fisher Scientific
Thermo Scientific Chemicals
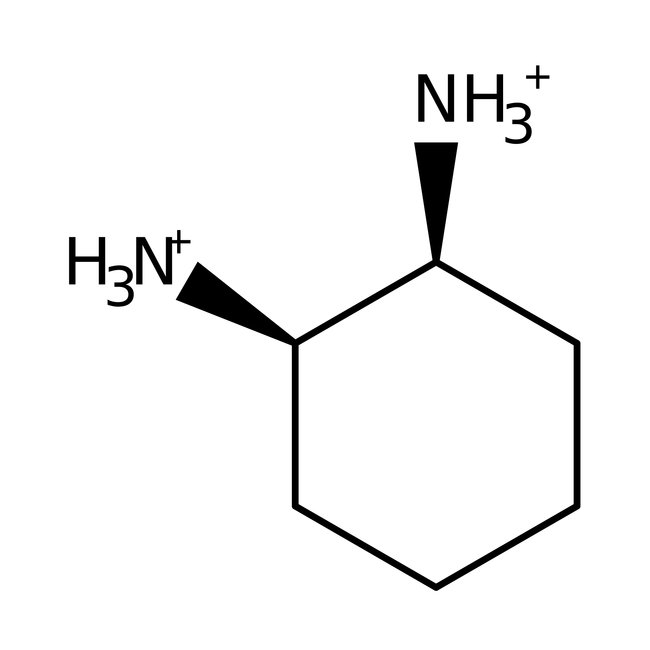
1,2-Diaminocyclohexane, mixture of isomers, 99%, Thermo Scientific Chemicals
CAS: 694-83-7 | C6H14N2 | 114.192 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFB24657.30 | 250 g |
Catalog number ALFB24657.30
Price (MYR)
409.00
Quantity:
250 g
Price (MYR)
409.00
Specifications
Chemical Name or Material1,2-Diaminocyclohexane
Name Notemixture of isomers
CAS694-83-7
Health Hazard 1H227-H302+H312+H332-H314-H335
Health Hazard 2GHS H Statement
H314-H318-H317-H227
Causes severe skin burns and eye damage.
Causes serious eye damage.
May cause an allergic skin reaction.
Combustible liquid.
H314-H318-H317-H227
Causes severe skin burns and eye damage.
Causes serious eye damage.
May cause an allergic skin reaction.
Combustible liquid.
View more
1,2-Diaminocyclohexane was used in the synthesis of chiral ruthenium(IV)-oxo complexes & bicyclic pyrazine derivatives.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
1,2-Diaminocyclohexane was used in the synthesis of chiral ruthenium(IV)-oxo complexes & bicyclic pyrazine derivatives.
Solubility
Fully miscible in water.
Notes
Store in a cool, dry place in tightly closed container. Incompatible with oxidizing agents, air, carbon dioxide.
1,2-Diaminocyclohexane was used in the synthesis of chiral ruthenium(IV)-oxo complexes & bicyclic pyrazine derivatives.
Solubility
Fully miscible in water.
Notes
Store in a cool, dry place in tightly closed container. Incompatible with oxidizing agents, air, carbon dioxide.
RUO – Research Use Only
General References:
- J J Bonire; S P Fricker. The in vitro antitumour profile of some 1,2-diaminocyclohexane organotin complexes. Journal of Inorganic Biochemistry. 2001, 83 (2-3), 217-221.
- Robert Häner; Florian Garo; Daniel Wenger; Vladimir L Malinovskii. Oligopyrenotides: abiotic, polyanionic oligomers with nucleic acid-like structural properties. Journal of the American Chemical Society. 2010, 132 (21), 7466-7471.