Search Thermo Fisher Scientific
Thermo Scientific Chemicals
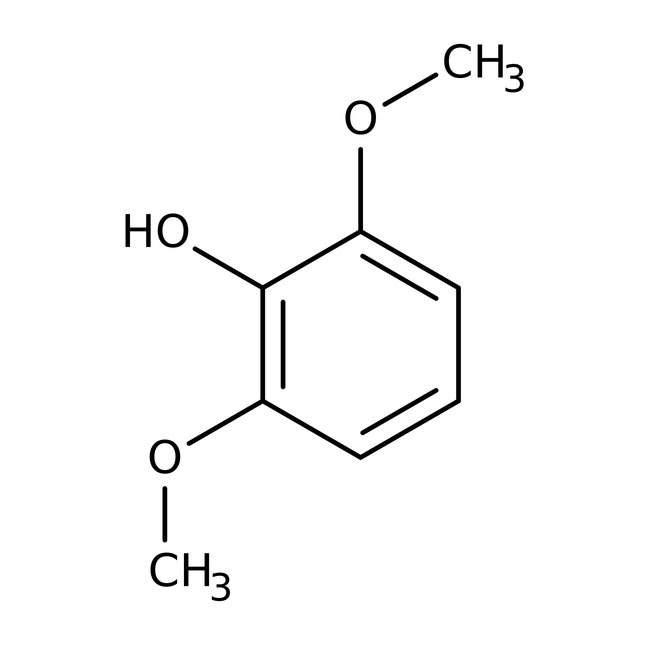
2,6-Dimethoxyphenol, 99%, Thermo Scientific Chemicals
CAS: 91-10-1 | C8H10O3 | 154.17 g/mol
Catalog number ALFB24253.14
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
25 g
Chemical Identifiers
CAS446-48-0
IUPAC Name1-(bromomethyl)-2-fluorobenzene
Molecular FormulaC7H6BrF
InChI KeyFFWQLZFIMNTUCZ-UHFFFAOYSA-N
SMILESFC1=CC=CC=C1CBr
View more
Specifications Specification Sheet
Specification Sheet
Appearance (Color)Clear colorless to light yellow
Appearance (Form)Liquid
Infrared spectrumConforms
GC>=97.5 %
Refractive index1.5495 to 1.5515 (20°C, 589 nm)
It is used in smoke flavors, whisky, rum, tea, spice, savory, seafood, meat, liquorices, coffee, and nut flavors. It is reported to be the single most important flavor chemical in smoke flavors.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
It is used in smoke flavors, whisky, rum, tea, spice, savory, seafood, meat, liquorices, coffee, and nut flavors. It is reported to be the single most important flavor chemical in smoke flavors.
Solubility
Soluble in water.
Notes
Store at room temperature. Incompatible with oxidizing agents.
It is used in smoke flavors, whisky, rum, tea, spice, savory, seafood, meat, liquorices, coffee, and nut flavors. It is reported to be the single most important flavor chemical in smoke flavors.
Solubility
Soluble in water.
Notes
Store at room temperature. Incompatible with oxidizing agents.
RUO – Research Use Only
General References:
- Hideo Kuwahara.; Ayako Kanazawa.; Daisuke Wakamatu.; Shigeru Morimura.; Kenji Kida.; Takaaki Akaike.; Hiroshi Maeda. Antioxidative and Antimutagenic Activities of 4-Vinyl-2,6-dimethoxyphenol (Canolol) Isolated from Canola Oil. J. Agric. Food Chem. 2004, 52 (14),4380-4387 .
- J.Chadwick Roper.; Jawed M. Sarkar.; Jerzy Dec.; Jean-Marc Bollag. Enhanced enzymatic removal of chlorophenols in the presence of co-substrates.Water Res. 1995, 29 (12),2720-2724 .
- For a study of the direct dilithiation of alkoxyphenols, see: J. Org. Chem., 55, 3902 (1990).