Search Thermo Fisher Scientific
Thermo Scientific Chemicals
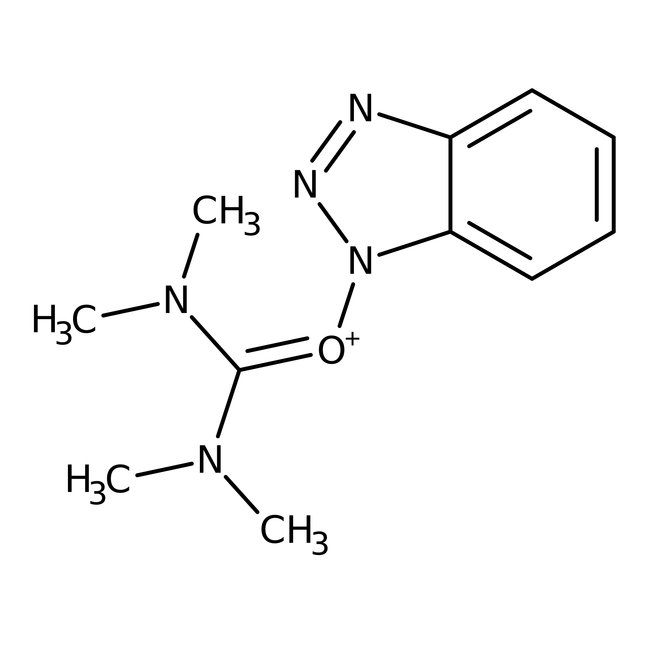
O-(1H-Benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate, 98%, Thermo Scientific Chemicals
CAS: 94790-37-1 | C11H16F6N5OP | 379.247 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFB23597.22 | 100 g |
Catalog number ALFB23597.22
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
100 g
Chemical Identifiers
CAS8013-01-2
Specifications Specification Sheet
Specification Sheet
FormPowder
Sodium Chloride≤0.5%
Total Nitrogen10-11.8%
Appearance (Color)Cream to pale yellow to pale brown
Loss on Drying≤8.0%
View more
O-(1H-Benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate is used as a coupling reagent for solid-phase peptide synthesis. It has been shown to effectively suppress racemization.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
O-(1H-Benzotriazol-1-yl)-N,N,N′,N′-tetramethyluronium hexafluorophosphate is used as a coupling reagent for solid-phase peptide synthesis. It has been shown to effectively suppress racemization.
Solubility
Soluble in acetonitrile.
Notes
Store in a cool place. Keep the container tightly closed in a dry and well-ventilated place. Incompatible with oxidizing agents.
O-(1H-Benzotriazol-1-yl)-N,N,N′,N′-tetramethyluronium hexafluorophosphate is used as a coupling reagent for solid-phase peptide synthesis. It has been shown to effectively suppress racemization.
Solubility
Soluble in acetonitrile.
Notes
Store in a cool place. Keep the container tightly closed in a dry and well-ventilated place. Incompatible with oxidizing agents.
RUO – Research Use Only
General References:
- Peptide coupling reagent giving high yields with minimal racemization: Synthesis, 572 (1984). See Appendix 6.
- Yoshida, M.; Sasahara, K. I.; Doi, T. Total synthesis of cyclodepsipeptide spiruchostatin A on silyl-linked polymer-support. Tetrahedron 2015, 71 (40), 7647-7653.
- Buba, A. E.; Löwe, H.; Kunz, H. Fluorenylmethoxycarbonyl-Protected O-Glycosyl-N-methyl Amino Acids: Building Blocks for the Synthesis of Conformationally Tuned Glycopeptide Antigens. Eur. J. Org. Chem. 2015, 2015 (26), 5764-5774.