Search Thermo Fisher Scientific
Thermo Scientific Chemicals
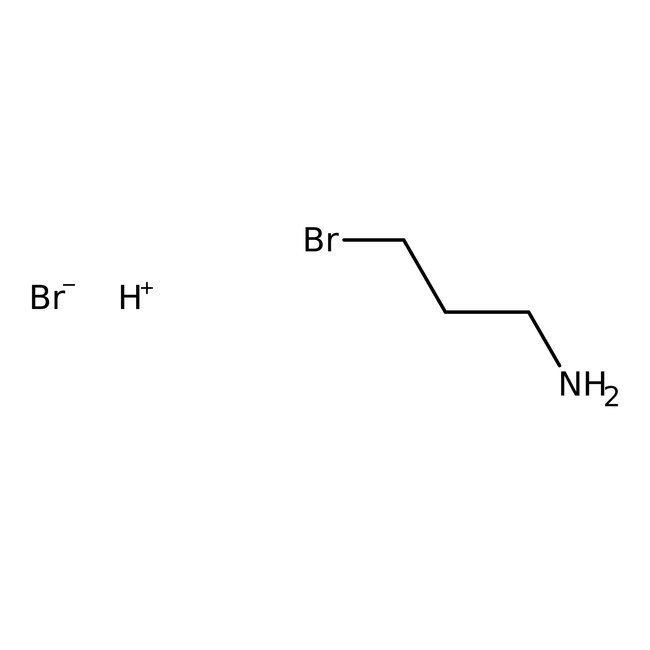
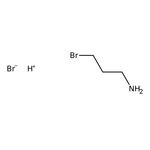
3-Bromopropylamine hydrobromide, 98%, Thermo Scientific Chemicals
CAS: 5003-71-4 | C3H9Br2N | 218.92 g/mol
Catalog number ALFB23254.22
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
100 g
Chemical Identifiers
CAS105931-73-5
IUPAC Name4-bromo-2-fluoro-1-iodobenzene
Molecular FormulaC6H3BrFI
InChI KeyXRMZKCQCINEBEI-UHFFFAOYSA-N
SMILESFC1=C(I)C=CC(Br)=C1
View more
Specifications Specification Sheet
Specification Sheet
Appearance (Form)Crystalline powder or crystals and/or chunks
Infrared spectrumConforms
Melting point46°C to 49°C
Appearance (Color)Off-white to beige
GC>=99.0 %
3-Bromopropylamine hydrobromide is used as pharmaceutical raw materials and intermediates. Homotaurine is synthesized from 3-bromopropylamine hydrobromide.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
3-Bromopropylamine hydrobromide is used as pharmaceutical raw materials and intermediates. Homotaurine is synthesized from 3-bromopropylamine hydrobromide.
Solubility
Soluble in water (50 mg/ml).
Notes
Hygroscopic. Store under inert gas. Store away from oxidizing agents, moisture.
3-Bromopropylamine hydrobromide is used as pharmaceutical raw materials and intermediates. Homotaurine is synthesized from 3-bromopropylamine hydrobromide.
Solubility
Soluble in water (50 mg/ml).
Notes
Hygroscopic. Store under inert gas. Store away from oxidizing agents, moisture.
RUO – Research Use Only
General References:
- Eleanor D. Bates; Rebecca D. Mayton; Ioanna Ntai and James H. Davis , Jr. CO2 Capture by a Task-Specific Ionic Liquid. J. Am. Chem. Soc. 2002, 124 (6), 926-927.
- R. F. Parcell; F. P. HauckJr. The Preparation of Tetrahydropyridines from Enamines and Imines. J. Org. Chem. 1963, 28 (12), 3468-3473.