Search Thermo Fisher Scientific
Thermo Scientific Chemicals
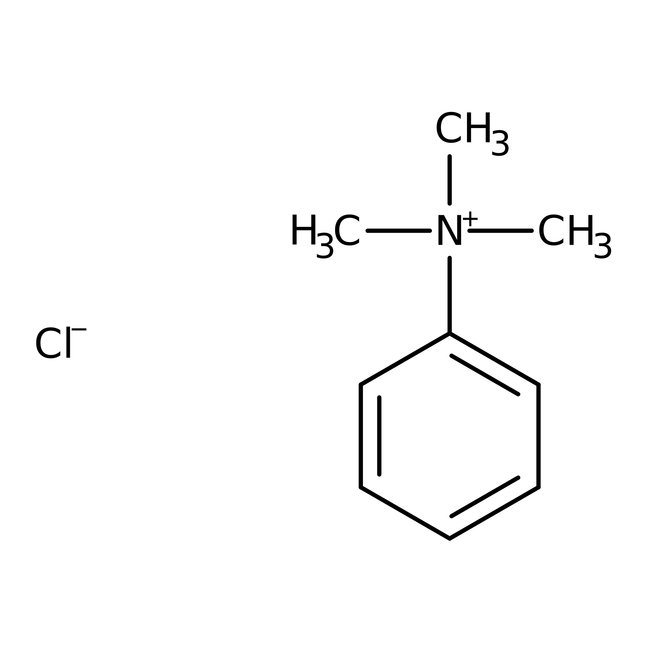
Phenyltrimethylammonium chloride, 98+%, Thermo Scientific Chemicals
CAS: 138-24-9 | C9H14ClN | 171.67 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFB22851.22 | 100 g |
Catalog number ALFB22851.22
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
100 g
Chemical Identifiers
CAS2595-97-3
IUPAC Name(3R,4R,5S,6R)-6-(hydroxymethyl)oxane-2,3,4,5-tetrol
Molecular FormulaC6H12O6
InChI KeyWQZGKKKJIJFFOK-IVMDWMLBSA-N
SMILESOC[C@H]1OC(O)[C@H](O)[C@H](O)[C@@H]1O
View more
Specifications Specification Sheet
Specification Sheet
Appearance (Color)White
FormCrystals or powder or crystalline powder
Assay (Silylated GC)≥97.0%
Water Content (Karl Fischer Titration)≤1.0%
Optical Rotation+15.0? ? 1.0? (c=5 in Distilled water)
Phenyltrimethylammonium chloride is an effective phase transfer catalyst, methylating agent, also useful for alkaline depolymerizations, corrosion inhibitor for low carbon steel.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Phenyltrimethylammonium chloride is an effective phase transfer catalyst, methylating agent, also useful for alkaline depolymerizations, corrosion inhibitor for low carbon steel.
Solubility
Soluble in water 333 g/L.
Notes
Hygroscopic. Store under inert gas. Store away from oxidizing agent, water/ moisture.
Phenyltrimethylammonium chloride is an effective phase transfer catalyst, methylating agent, also useful for alkaline depolymerizations, corrosion inhibitor for low carbon steel.
Solubility
Soluble in water 333 g/L.
Notes
Hygroscopic. Store under inert gas. Store away from oxidizing agent, water/ moisture.
RUO – Research Use Only
General References:
- Paul G. Sears; Eugene D. Wilhoit and Lyle R. Dawson. Conductances of Trimethylphenylammonium Chloride and Iodide in Water and in Dimethylformamide at 25°C. J. Chem. Phys. 1955, 23 (7), 1274.
- T. Jiang; M.J. Chollier Brym; G. Dubéb, A. Lasia; G.M. Brisard. Electrodeposition of Aluminum from ionic liquids: Part II - studies on the electrodeposition of aluminum from aluminum chloride (AICl3) - trimethylphenylammonium chloride (TMPAC) ionic liquids. Surface and Coatings Technology. 2006, 201, (1-2), 10-18.