Search Thermo Fisher Scientific
Thermo Scientific Chemicals
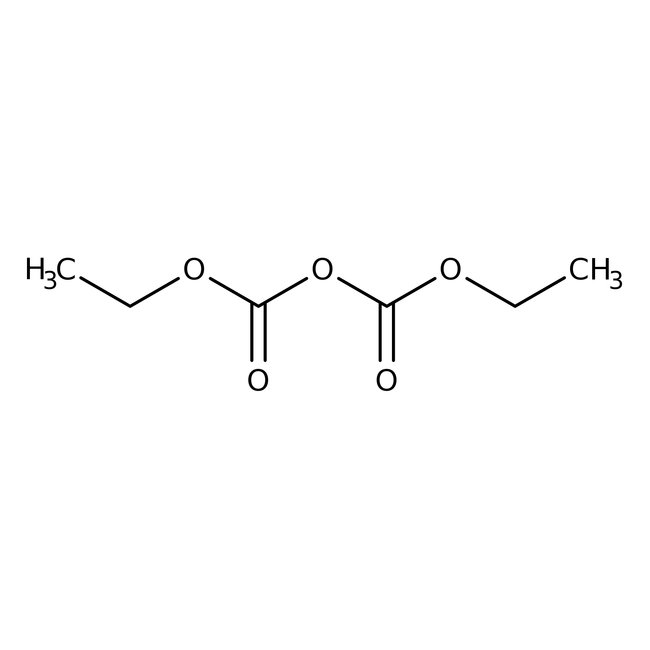
Diethyl dicarbonate, 97%, Thermo Scientific Chemicals
CAS: 1609-47-8 | C6H10O5 | 162.14 g/mol
Catalog number ALFB22753.06
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
5 g
Specifications
Chemical Name or MaterialDiethyl dicarbonate
CAS1609-47-8
Health Hazard 1H227-H302-H315-H319-H332-H335
Health Hazard 2GHS H Statement
H302-H315-H319-H335-H227
Harmful if swallowed.
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
Combustible liquid.
H302-H315-H319-H335-H227
Harmful if swallowed.
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
Combustible liquid.
Health Hazard 3P210-P235-P261-P264b-P270-P271-P280-P301+P312-P302+P352-P304+P340-P305+P351+P338-P312-P330-P332+P313-P362-P370+P378q-P501c
View more
Ribonuclease inhibitorDiethyl dicarbonate acts as an inhibitor of ryanodine binding to ryanodine/calcium(II) receptor channel. It is useful for specific inactivation of nucleases during isolation of undegraded polynucleotides. Further, it inhibits platelet-activating factor acetyl hydrolase. In addition to this, it is involved in the modification reagent for His and Tyr residues in proteins.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Ribonuclease inhibitorDiethyl dicarbonate acts as an inhibitor of ryanodine binding to ryanodine/calcium(II) receptor channel. It is useful for specific inactivation of nucleases during isolation of undegraded polynucleotides. Further, it inhibits platelet-activating factor acetyl hydrolase. In addition to this, it is involved in the modification reagent for His and Tyr residues in proteins.
Solubility
Miscible with chloroform, ethanol, esters, ketones and hydrocarbons. Slightly miscible with water.
Notes
Store in cool place. Incompatible with strong oxidizing agents, strong reducing agents, strong acids, strong bases and ammonia.
Ribonuclease inhibitorDiethyl dicarbonate acts as an inhibitor of ryanodine binding to ryanodine/calcium(II) receptor channel. It is useful for specific inactivation of nucleases during isolation of undegraded polynucleotides. Further, it inhibits platelet-activating factor acetyl hydrolase. In addition to this, it is involved in the modification reagent for His and Tyr residues in proteins.
Solubility
Miscible with chloroform, ethanol, esters, ketones and hydrocarbons. Slightly miscible with water.
Notes
Store in cool place. Incompatible with strong oxidizing agents, strong reducing agents, strong acids, strong bases and ammonia.
RUO – Research Use Only
General References:
- In the presence of the hindered base lithium dicyclohexylamide (from Dicyclohexyl amine, A15671), ɑ -ethoxycarbonylation of ketones occurs to give ß -keto esters: Synthesis, 1014 (1984).
- Dogandzhiyski, P.; Ghidini, A.; Danneberg, F.; Strömberg, R.; Göbel, M. W. Studies on Tris(2-aminobenzimidazole)-PNA Based Artificial Nucleases: A Comparison of Two Analytical Techniques. Bioconjugate Chem. 2015, 26 (12), 2514-2519.
- Zuldesmi, M.; Waki, A.; Kuroda, K.; Okido, M. Hydrothermal treatment of titanium alloys for the enhancement of osteoconductivity. Mater. Sci. Eng., C 2015, 49, 430-435.