Search Thermo Fisher Scientific
Thermo Scientific Chemicals
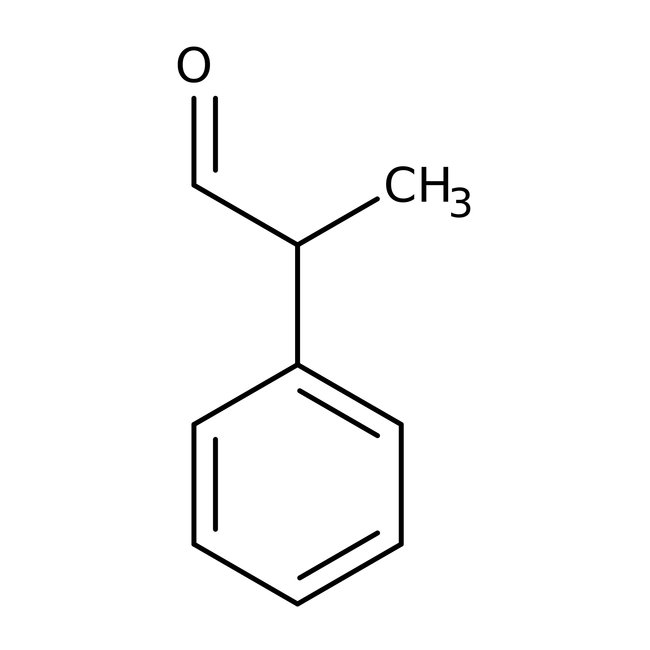
2-Phenylpropionaldehyde, 97%, Thermo Scientific Chemicals
CAS: 93-53-8 | C9H10O | 134.178 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFB22436.22 | 100 g |
Catalog number ALFB22436.22
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
100 g
Specifications
Chemical Name or Material2-Phenylpropionaldehyde
CAS93-53-8
Health Hazard 1H227-H315-H317-H319-H361f
Health Hazard 2GHS H Statement
H315-H319-H335-H227
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
Combustible liquid.
H315-H319-H335-H227
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
Combustible liquid.
Health Hazard 3P201-P202-P210-P261-P264b-P272-P280-P281-P302+P352-P305+P351+P338-P308+P313-P333+P313-P362-P370+P378q-P501c
View more
2-phenylpropionaldehyde to give acetophenone using 1 atm molecular oxygen as oxidant was catalyzed by Ce(IV), V(IV) and Bronsted acids. It is used to produce 3-hydroxy-2-methyl-2-phenyl-propionaldehyde. This reaction will need reagent water and K2CO3.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
2-phenylpropionaldehyde to give acetophenone using 1 atm molecular oxygen as oxidant was catalyzed by Ce(IV), V(IV) and Bronsted acids. It is used to produce 3-hydroxy-2-methyl-2-phenyl-propionaldehyde. This reaction will need reagent water and K2CO3.
Solubility
Soluble in most fixed oils, propylene glycol. Insoluble in glycerin.
Notes
Air Sensitive. Keep container tightly closed. Store in cool, dry conditions in well sealed containers.
2-phenylpropionaldehyde to give acetophenone using 1 atm molecular oxygen as oxidant was catalyzed by Ce(IV), V(IV) and Bronsted acids. It is used to produce 3-hydroxy-2-methyl-2-phenyl-propionaldehyde. This reaction will need reagent water and K2CO3.
Solubility
Soluble in most fixed oils, propylene glycol. Insoluble in glycerin.
Notes
Air Sensitive. Keep container tightly closed. Store in cool, dry conditions in well sealed containers.
RUO – Research Use Only
General References:
- Richard F. Heck. The palladium-catalyzed arylation of enol esters, ethers, and halides. A new synthesis of 2-aryl aldehydes and ketones. J. Am. Chem. Soc. 1968, 90, (20), 5535-5538.
- Charles D. Thompson.; Michael T. Kinter.; Timothy L. Macdonald. Synthesis and in Vitro Reactivity of 3-Carbamoyl-2-phenylpropionaldehyde and 2-Phenylpropenal: Putative Reactive Metabolites of Felbamate. Chem. Res. Toxicol.1996, 9, (8), 1225-1229.