Search Thermo Fisher Scientific
Thermo Scientific Chemicals
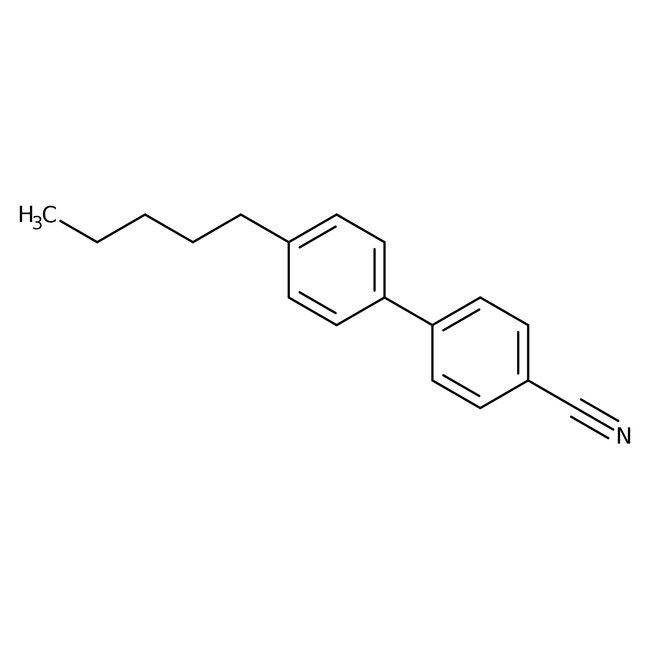
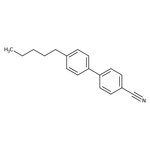
4-Cyano-4'-n-pentylbiphenyl, 99%, Thermo Scientific Chemicals
CAS: 40817-08-1 | C18H19N | 249.357 g/mol
Catalog number ALFB21856.03
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
1 g
Chemical Identifiers
CAS108-31-6
IUPAC Name2,5-dihydrofuran-2,5-dione
Molecular FormulaC4H2O3
InChI KeyFPYJFEHAWHCUMM-UHFFFAOYSA-N
SMILESO=C1OC(=O)C=C1
View more
Specifications Specification Sheet
Specification Sheet
Infrared spectrumConforms
Appearance (Form)Pastilles
Melting point52°C to 55°C
Free acid=<0.5 % (as maleic acid)
Appearance (Color)White
View more
4-Cyano-4'-n-pentylbiphenyl is used in the preparation of 4'-pentylbiphenyl-4-carboxylic acid by acidic hydrolysis using sulfuric acid as a reagent.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
4-Cyano-4′-n-pentylbiphenyl is used in the preparation of 4′-pentylbiphenyl-4-carboxylic acid by acidic hydrolysis using sulfuric acid as a reagent.
Solubility
Miscible with Acetone, chloroform, toluene, acetonitrile, methylene chloride. Immiscible with water.
Notes
Hygroscopic. Incompatible with strong oxidizing agents.
4-Cyano-4′-n-pentylbiphenyl is used in the preparation of 4′-pentylbiphenyl-4-carboxylic acid by acidic hydrolysis using sulfuric acid as a reagent.
Solubility
Miscible with Acetone, chloroform, toluene, acetonitrile, methylene chloride. Immiscible with water.
Notes
Hygroscopic. Incompatible with strong oxidizing agents.
RUO – Research Use Only
General References:
- Matsushita, S.; Yan, B.; Yamamoto, S.; Jeong, Y. S.; Akagi, K. Helical Carbon and Graphite Films Prepared from Helical Poly(3,4-ethylenedioxythiophene) Films Synthesized by Electrochemical Polymerization in Chiral Nematic Liquid Crystals. Angew. Chem. Int. Ed. 2014, 53 (6), 1659-1663.
- Kasch, N.; Dierking, I.; Turner, M.; Romero-Hasler, P.; Soto-Bustamante, E. A. Liquid crystalline textures and polymer morphologies resulting from electropolymerisation in liquid crystal phases. J. Mater. Chem. C 2015, 3 (31), 8018-8023.