Search Thermo Fisher Scientific
Thermo Scientific Chemicals
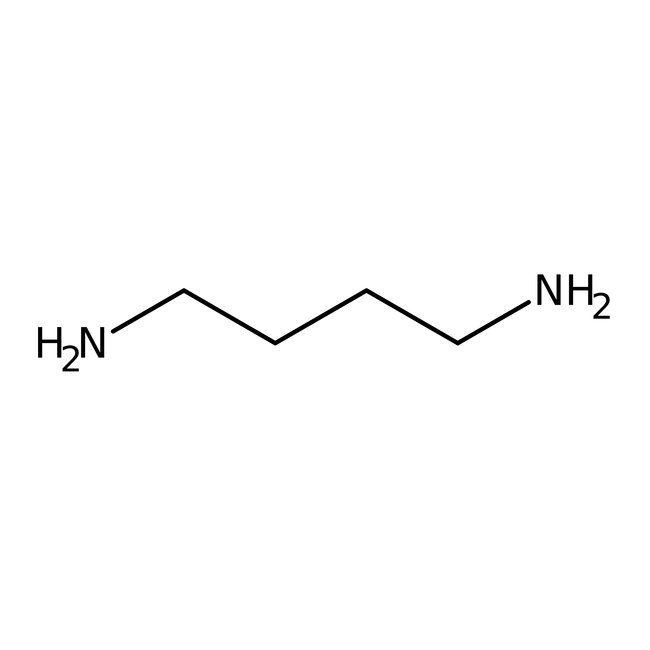
1,4-Diaminobutane, 98+%, Thermo Scientific Chemicals
CAS: 110-60-1 | C4H12N2 | 88.154 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFB21316.14 | 25 g |
Catalog number ALFB21316.14
Price (MYR)
268.00
Quantity:
25 g
Price (MYR)
268.00
Specifications
Chemical Name or Material1,4-Diaminobutane
CAS110-60-1
Health Hazard 1H302-H311-H314-H330-H335
Health Hazard 2GHS H Statement
H331-H314-H318-H302-H312
Toxic if inhaled.
Causes severe skin burns and eye damage.
Causes serious eye damage.
Harmful if swallowed.
Harmful in contact with skin.
H331-H314-H318-H302-H312
Toxic if inhaled.
Causes severe skin burns and eye damage.
Causes serious eye damage.
Harmful if swallowed.
Harmful in contact with skin.
Health Hazard 3P260-P264b-P270-P271-P280-P284-P303+P361+P353-P304+P340-P305+P351+P338-P310-P330-P331-P363-P501c
View more
1,4-Diaminobutane is used as a precursor in many biological systems and synthon for amido-ureas. It is involved in the synthesis of nylon 46 by reacting with adipic acid.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
1,4-Diaminobutane is used as a precursor in many biological systems and synthon for amido-ureas. It is involved in the synthesis of nylon 46 by reacting with adipic acid.
Solubility
Soluble in ether and alcohol.
Notes
Incompatible with acids, strong oxidizing agents, aldehydes, acid anhydrides and acid chlorides.
1,4-Diaminobutane is used as a precursor in many biological systems and synthon for amido-ureas. It is involved in the synthesis of nylon 46 by reacting with adipic acid.
Solubility
Soluble in ether and alcohol.
Notes
Incompatible with acids, strong oxidizing agents, aldehydes, acid anhydrides and acid chlorides.
RUO – Research Use Only
General References:
- Ahmed, N. C-B.; Negadi, L.; Mokbel, I.; Jose, J. Phase equilibrium properties of binary aqueous solutions containing ethanediamine, 1,2-diaminopropane, 1,3-diaminopropane, or 1,4-diaminobutane at several temperatures. J. Chem. Thermodyn. 2011,43 (5), 719-724.
- Kim, Y. E.; Yun, S. H.; Choi, J. H.; Nam, S. C.; Park, S. Y.; Jeong, S. K.; Yoon, Y. Comparison of the CO2 Absorption Characteristics of Aqueous Solutions of Diamines: Absorption Capacity, Specific Heat Capacity, and Heat of Absorption. Energy Fuels. 2015, 29 (4), 2582-2590.
- Hoang, G. T.; Kubo, T.; Young, V. G.; Kautzky, J. A.; Wissinger, J. E. Illustrating the Utility of X-ray Crystallography for Structure Elucidation through a Tandem Aldol Condensation/Diels-Alder Reaction Sequence. J. Chem. Educ. 2015, 92 (8), 1381-1384.