Search Thermo Fisher Scientific
Thermo Scientific Chemicals
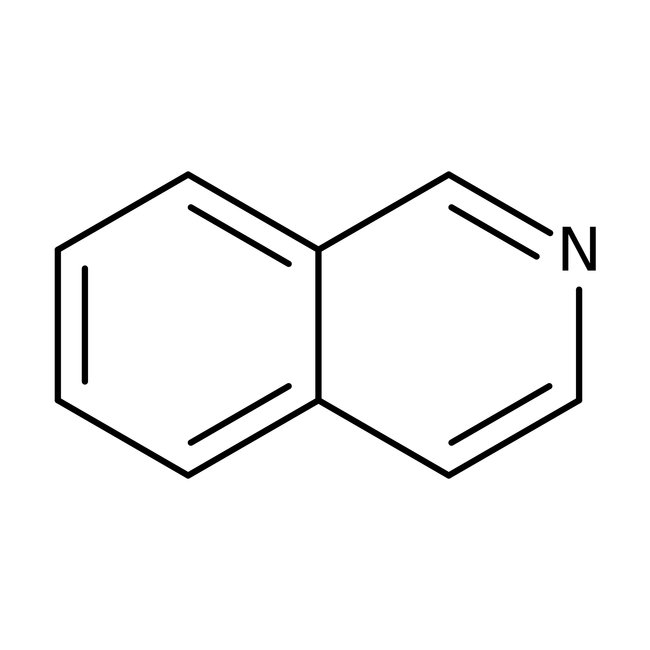
Isoquinoline, 97%, Thermo Scientific Chemicals
| Catalog Number | Quantity |
|---|---|
| ALFB21279.36 | 500 g |
Catalog number ALFB21279.36
Price (MYR)
2,127.00
Quantity:
500 g
Price (MYR)
2,127.00
Specifications
Chemical Name or MaterialIsoquinoline
CAS119-65-3
Health Hazard 1H302-H310-H315-H319
Health Hazard 2GHS H Statement
H311-H302-H315-H319
Toxic in contact with skin.
Harmful if swallowed.
Causes skin irritation.
Causes serious eye irritation.
H311-H302-H315-H319
Toxic in contact with skin.
Harmful if swallowed.
Causes skin irritation.
Causes serious eye irritation.
Health Hazard 3P261-P262-P264b-P270-P280-P301+P312-P302+P352-P305+P351+P338-P310-P330-P362-P501c
View more
Isoquinoline is used in the manufacture of dyes, paints, antifungal. It is also used as a solvent for the extraction of resins and terpenes.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Isoquinoline is used in the manufacture of dyes, paints, antifungal. It is also used as a solvent for the extraction of resins and terpenes.
Solubility
Soluble in water (5g/L).
Notes
Store away from strong oxidizing agents. Keep container tightly closed. Store in cool, dry conditions in well sealed containers.
Isoquinoline is used in the manufacture of dyes, paints, antifungal. It is also used as a solvent for the extraction of resins and terpenes.
Solubility
Soluble in water (5g/L).
Notes
Store away from strong oxidizing agents. Keep container tightly closed. Store in cool, dry conditions in well sealed containers.
RUO – Research Use Only
General References:
- Maria Chrzanowska.; Maria D. Rozwadowska. Asymmetric Synthesis of Isoquinoline Alkaloids. Chem. Rev. 2004, 104, (7), 3341-3370.
- Ryoji. Noyori.; Masako. Ohta.; Yi. Hsiao.; Masato. Kitamura.; Tetsuo. Ohta.; Hidemasa. Takaya. Asymmetric synthesis of isoquinoline alkaloids by homogeneous catalysis. J. Am. Chem. Soc. 1986, 108, (22), 7117-7119.
- Reacts with various acid halides in the presence of CN- to give Reissert compounds (1-cyano-2-acyl 1,2-dihydro derivatives). For reviews, see: Chem. Rev., 55, 511 (1955); Adv. Het. Chem., 9, 1 (1968); 24, 187 (1979). 1-Alkylisoquinolines can be prepared by alkylation of the Li or Na derivatives of Reissert compounds: Org. Synth. Coll., 4, 641 (1963); 6, 115 (1988). For formation of Reissert compounds from a variety of carbonyl, sulfonyl and phosphoryl halides under milder, phase-transfer conditions, see: Synthesis, 497 (1977). For 'direct' cyanation by a modified Reissert procedure in the presence of tosyl chloride followed by DBU, see: J. Org. Chem., 49, 4056 (1984).
- A one-step synthesis of 1-nitroisoquinoline has been reported in which isoquinoline reacts with KNO2 in the presence of acetic anhydride and DMSO. The reaction is considered to be analogous to the Pfitzner-Moffatt oxidation (see Dimethyl sulfoxide, A13280): J. Chem. Soc., Perkin 1, 1777 (1996).