Search Thermo Fisher Scientific
Thermo Scientific Chemicals
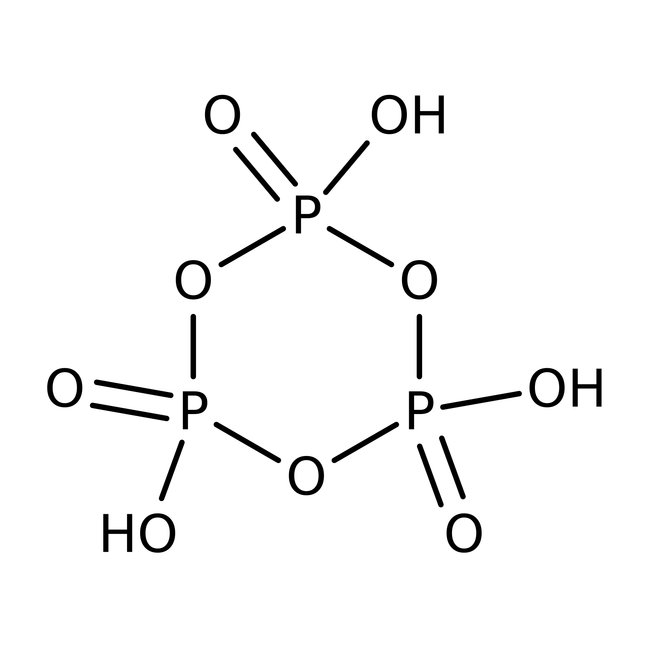
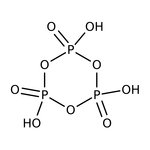
Metaphosphoric acid, 39-43%, bal. NaPO{3} (Stabilizer), Thermo Scientific Chemicals
CAS: 37267-86-0 | HO3P | 79.979 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFB20932.22 | 100 g |
Catalog number ALFB20932.22
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
100 g
Chemical Identifiers
CAS620-13-3
IUPAC Name1-(bromomethyl)-3-methylbenzene
Molecular FormulaC8H9Br
InChI KeyFWLWTILKTABGKQ-UHFFFAOYSA-N
SMILESCC1=CC=CC(CBr)=C1
View more
Specifications Specification Sheet
Specification Sheet
Appearance (Color)Clear colorless to yellow
FormLiquid
Assay (GC)≥96.0%
Refractive Index1.5640-1.5690 @ 20°C
Metaphosphoric acid is used as a dehydrating agent. Its derivative, sodium hexametaphosphate is used as a sequestrant and a food additive. It is also used as a phosphorylating agent, analytical reagent and in dental cements.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Metaphosphoric acid is used as a dehydrating agent. Its derivative, sodium hexametaphosphate is used as a sequestrant and a food additive. It is also used as a phosphorylating agent, analytical reagent and in dental cements.
Solubility
Soluble in alcohol.
Notes
Form: Lumps.Hygroscopic. Moisture sensitive. Incompatible with strong bases, nitromethane and metals.
Metaphosphoric acid is used as a dehydrating agent. Its derivative, sodium hexametaphosphate is used as a sequestrant and a food additive. It is also used as a phosphorylating agent, analytical reagent and in dental cements.
Solubility
Soluble in alcohol.
Notes
Form: Lumps.Hygroscopic. Moisture sensitive. Incompatible with strong bases, nitromethane and metals.
RUO – Research Use Only
General References:
- Alkorta, I.; Azofra, L. M.; Elguero, J. Ab initio study in the hydration process of metaphosphoric acid: the importance of the pnictogen interactions. Theor. Chem. Acc. 2015, 134 (3), 1-8.
- Feitosa, V. P.; Bazzocchi, M. G.; Putignano, A.; Orsini, G.; Luzi, A. L.; Sinhoreti, M. A. C.; Watson, T. F.; Sauro, S. Dicalcium phosphate(CaHPO4·2H2O) precipitation through ortho- or meta-phosphoric acid-etching: Effects on the durability and nanoleakage/ultra-morphology of resin-dentine interfaces. J. Dent. 2013, 41 (11), 1068-1080.