Search Thermo Fisher Scientific
Thermo Scientific Chemicals
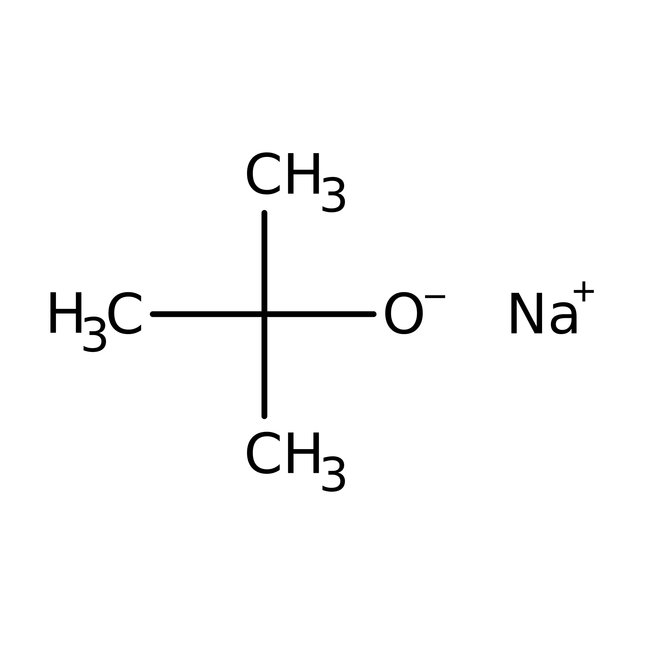
Sodium tert-butoxide, 97%, Thermo Scientific Chemicals
CAS: 865-48-5 | C4H9NaO | 96.105 g/mol
Catalog number ALFA19942.36
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
500 g
Chemical Identifiers
CAS79-14-1
IUPAC Name2-hydroxyacetic acid
Molecular FormulaC2H4O3
InChI KeyAEMRFAOFKBGASW-UHFFFAOYSA-N
SMILESOCC(O)=O
View more
Specifications Specification Sheet
Specification Sheet
Appearance (Color)White to off-white
Infrared spectrumConforms
Titration with NaOH>=98.5 % (On dry substance)
Appearance (Form)Adhering crystals
Water=<1 %
View more
Sodium tert-butoxide is used as a strong base and a non-nucleophilic base. In organic synthesis, it serves as an intermediate in various reactions like condensation, rearrangement and ring-opening. Further, it finds use in agrochemicals, pharmaceuticals, colorants, aroma chemicals, detergents and biodiesel. It acts as a catalyst in polymerization and isomerization reactions.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Sodium tert-butoxide is used as a strong base and a non-nucleophilic base. In organic synthesis, it serves as an intermediate in various reactions like condensation, rearrangement and ring-opening. Further, it finds use in agrochemicals, pharmaceuticals, colorants, aroma chemicals, detergents and biodiesel. It acts as a catalyst in polymerization and isomerization reactions.
Notes
Air sensitive and hygroscopic. Incompatible with acids, reducing agents, oxygen, water, alcohols, chlorinated solvents and halogens.
Sodium tert-butoxide is used as a strong base and a non-nucleophilic base. In organic synthesis, it serves as an intermediate in various reactions like condensation, rearrangement and ring-opening. Further, it finds use in agrochemicals, pharmaceuticals, colorants, aroma chemicals, detergents and biodiesel. It acts as a catalyst in polymerization and isomerization reactions.
Notes
Air sensitive and hygroscopic. Incompatible with acids, reducing agents, oxygen, water, alcohols, chlorinated solvents and halogens.
RUO – Research Use Only
General References:
- Preferred base, superior to KO-t-Bu or LiO-t-Bu, for promoting Buchwald's Pd catalyzed conversion of aryl bromides to arylamines: Angew. Chem. Int. Ed., 34, 1316, 1348 (1995); J. Org. Chem., 61, 7240 (1996); Tetrahedron Lett., 39, 2219 (1998).
- Zhang, M. X.; Hu, X. H.; Xu, Y. H.; Loh, T. P. Selective Dealkylation of Alkyl Aryl Ethers. Asian J. Org. Chem. 2015, 4 (10), 1047-1049.
- Kumar, C. V.; Raptis, D.; Koukaras, E. N.; Sygellou, L.; Lianos, P. Study of an indoline-phenothiazine based organic dye for Dye-Sensitized Solar Cells. Theoretical calculations and experimental data. Org. Electron. 2015, 25, 66-73.