Search Thermo Fisher Scientific
Thermo Scientific Chemicals
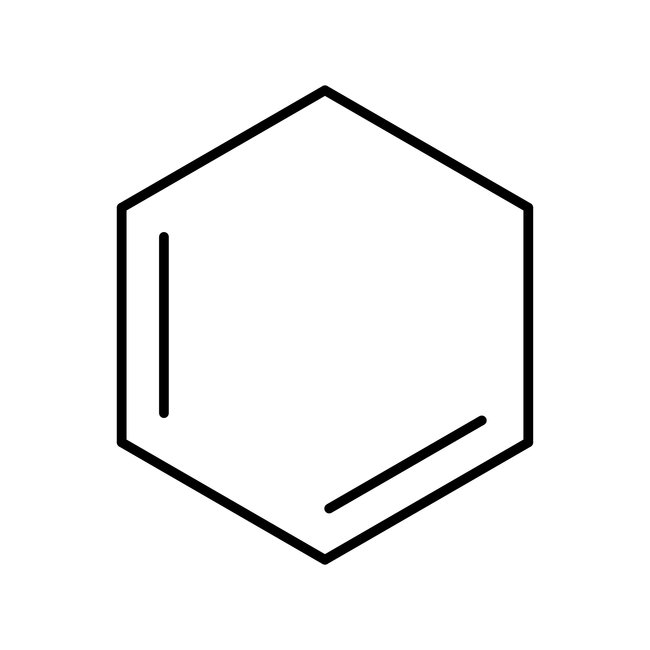
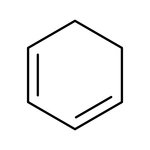
1,3-Cyclohexadiene, 96%, stab. with 0.1% BHT, Thermo Scientific Chemicals
CAS: 592-57-4 | C6H8 | 80.13 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFA19394.14 | 25 g |
Catalog number ALFA19394.14
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
25 g
Chemical Identifiers
CAS12125-02-9
IUPAC Nameammonium chloride
Molecular FormulaClH4N
InChI KeyNLXLAEXVIDQMFP-UHFFFAOYSA-N
SMILES[NH4+].[Cl-]
View more
Specifications Specification Sheet
Specification Sheet
Titration Argentometric>=99.5 %
Heavy metals (as Pb)=<5 ppm
Sulfated ash=<0.01 %
Insoluble matter=<0.005 %
pH4.5 to 5.5 (5 % at 20°C)
View more
1,3-Cyclohexadiene is used as a hydrogen donor in transfer hydrogenation. It is used in the conversion to benzene. It is useful in the study of proteomics research.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
1,3-Cyclohexadiene is used as a hydrogen donor in transfer hydrogenation. It is used in the conversion to benzene. It is useful in the study of proteomics research.
Solubility
Slightly miscible with methanol. Immiscible with water.
Notes
Incompatible with strong oxidizing agents. Store in a cool place.
1,3-Cyclohexadiene is used as a hydrogen donor in transfer hydrogenation. It is used in the conversion to benzene. It is useful in the study of proteomics research.
Solubility
Slightly miscible with methanol. Immiscible with water.
Notes
Incompatible with strong oxidizing agents. Store in a cool place.
RUO – Research Use Only
General References:
- Ohta, A.; Kobayashi, O.; Danielache, S. O.; Nanbu, S. Nonadiabatic ab initio molecular dynamics of photoisomerization reaction between 1, 3-cyclohexadiene and 1, 3, 5-cis-hexatriene. Chem. Phys. 2015, 459, 45-53.
- Adachi, S.; Sato, M.; Suzuki, T. Direct Observation of Ground-State Product Formation in a 1,3-Cyclohexadiene Ring-Opening Reaction. J. Phys. Chem. Lett. 2015, 6 (3), 343-346.