Search Thermo Fisher Scientific
Thermo Scientific Chemicals
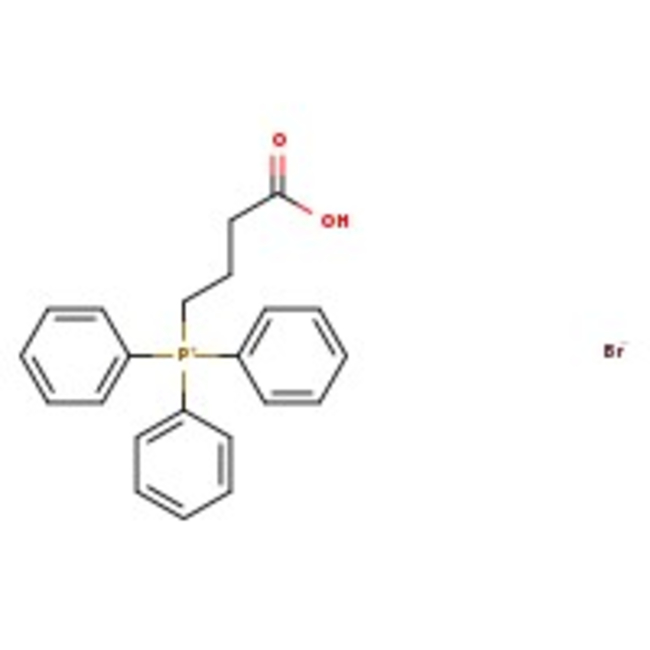
(3-Carboxypropyl)triphenylphosphonium bromide, 97%, Thermo Scientific Chemicals
CAS: 17857-14-6 | C22H22BrO2P | 429.29 g/mol
Catalog number ALFA19302.22
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
100 g
Specifications
Chemical Name or Material(3-Carboxypropyl)triphenylphosphonium bromide
CAS17857-14-6
Health Hazard 1H315-H319-H335
Health Hazard 2GHS H Statement
H315-H319-H335
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
H315-H319-H335
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
Health Hazard 3P261-P264b-P271-P280-P302+P352-P304+P340-P305+P351+P338-P312-P332+P313-P362-P501c
View more
Reactant for preparation of Piperamide analogs as histone deacetylase (HDAC) inhibitors with antitumor activity, Boron-containing benzoxaboroles as antimalarial agents, Methyl alkenyl quinolones as antimycobacterial agents, Solandelactone E via Sharpless epoxidation, Taber cyclopropanation, chemoselective reductions and lithiation-borylation-allylation sequence, Organotin compounds as antifungal agents, Oleanolic acid derivatives as inhibitors of protein tyrosine phosphatase 1B for negative regulation of insulin pathway (a promising target for treatment of diabetes and obesity), 11-oxa prostaglandin analogs with ocular hypotensive activity.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Reactant for preparation of Piperamide analogs as histone deacetylase (HDAC) inhibitors with antitumor activity, Boron-containing benzoxaboroles as antimalarial agents, Methyl alkenyl quinolones as antimycobacterial agents, Solandelactone E via Sharpless epoxidation, Taber cyclopropanation, chemoselective reductions and lithiation-borylation-allylation sequence, Organotin compounds as antifungal agents, Oleanolic acid derivatives as inhibitors of protein tyrosine phosphatase 1B for negative regulation of insulin pathway (a promising target for treatment of diabetes and obesity), 11-oxa prostaglandin analogs with ocular hypotensive activity.
Solubility
Soluble in water. soluble in ethanol.
Notes
Hygroscopic. Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Store away from strong oxidizing agents, water, moisture.
Reactant for preparation of Piperamide analogs as histone deacetylase (HDAC) inhibitors with antitumor activity, Boron-containing benzoxaboroles as antimalarial agents, Methyl alkenyl quinolones as antimycobacterial agents, Solandelactone E via Sharpless epoxidation, Taber cyclopropanation, chemoselective reductions and lithiation-borylation-allylation sequence, Organotin compounds as antifungal agents, Oleanolic acid derivatives as inhibitors of protein tyrosine phosphatase 1B for negative regulation of insulin pathway (a promising target for treatment of diabetes and obesity), 11-oxa prostaglandin analogs with ocular hypotensive activity.
Solubility
Soluble in water. soluble in ethanol.
Notes
Hygroscopic. Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Store away from strong oxidizing agents, water, moisture.
RUO – Research Use Only
General References:
- Yu Luo et. al. Synthesis and biological evaluation of piperamide analogues as HDAC inhibitors. Bioorganic & Medicinal Chemistry Letters,. 2011, 21 (16), 4844-4846 .
- Yong-Kang Zhang et. al. Synthesis and structure-activity relationships of novel benzoxaboroles as a new class of antimalarial agents. Bioorganic & Medicinal Chemistry Letters,. 2011, 21(2), 644-651 .