Search Thermo Fisher Scientific
Thermo Scientific Chemicals
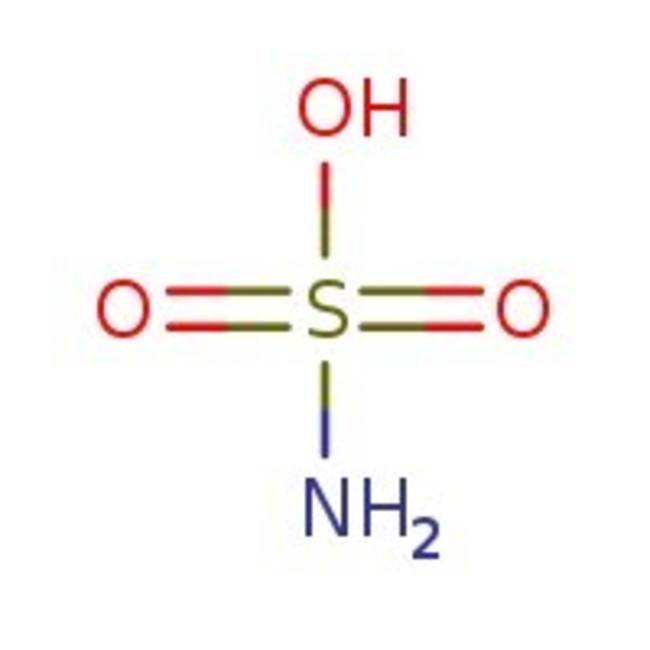
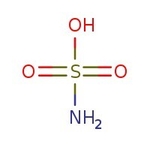
Amidosulfonic acid, 98+%, Thermo Scientific Chemicals
CAS: 5329-14-6 | H3NO3S | 97.088 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFA18836.22 | 100 g |
Catalog number ALFA18836.22
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
100 g
Chemical Identifiers
CAS645-49-8
IUPAC Name[(1Z)-2-phenylethenyl]benzene
Molecular FormulaC14H12
InChI KeyPJANXHGTPQOBST-QXMHVHEDSA-N
SMILESC(=CC1=CC=CC=C1)C1=CC=CC=C1
View more
Specifications Specification Sheet
Specification Sheet
Assay (GC)≥96.0%
FormLiquid
Refractive Index1.6185-1.6240 @ 20?C
Identification (FTIR)Conforms
Appearance (Color)Clear colorless to pale yellow
Amidosulfonic acid is widely utilized as a green catalyst for the preparation of amide from ketoxime. It finds an application as a titrant in the determination of the burette injection volume and chemical calibration factor. It is an efficient catalyst in the synthesis of polyhydroquinoline derivatives by Hantzsch condensation reaction, deazaoxaflavin, fatty acid methyl and ethyl esters and in the isolation of 5-hydroxymethylfurfural (HMF) from bamboo fiber. It is involved in the determination of silicates in water samples. It acts as a neutralizing agent during the determination of paracetamol by Glynn and Kendal colorimetric method.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Amidosulfonic acid is widely utilized as a green catalyst for the preparation of amide from ketoxime. It finds an application as a titrant in the determination of the burette injection volume and chemical calibration factor. It is an efficient catalyst in the synthesis of polyhydroquinoline derivatives by Hantzsch condensation reaction, deazaoxaflavin, fatty acid methyl and ethyl esters and in the isolation of 5-hydroxymethylfurfural (HMF) from bamboo fiber. It is involved in the determination of silicates in water samples. It acts as a neutralizing agent during the determination of paracetamol by Glynn and Kendal colorimetric method.
Solubility
Slightly soluble in water, dimethyl fluoride and methanol. Insoluble in hydrocarbons.
Notes
Incompatible with chlorine compounds, nitrates, carbonates, strong oxidizing agents and strong bases.
Amidosulfonic acid is widely utilized as a green catalyst for the preparation of amide from ketoxime. It finds an application as a titrant in the determination of the burette injection volume and chemical calibration factor. It is an efficient catalyst in the synthesis of polyhydroquinoline derivatives by Hantzsch condensation reaction, deazaoxaflavin, fatty acid methyl and ethyl esters and in the isolation of 5-hydroxymethylfurfural (HMF) from bamboo fiber. It is involved in the determination of silicates in water samples. It acts as a neutralizing agent during the determination of paracetamol by Glynn and Kendal colorimetric method.
Solubility
Slightly soluble in water, dimethyl fluoride and methanol. Insoluble in hydrocarbons.
Notes
Incompatible with chlorine compounds, nitrates, carbonates, strong oxidizing agents and strong bases.
RUO – Research Use Only
General References:
- Widely used to destroy excess HNO2 in diazotization reactions. Effective catalyst for acetylation of alcohols and phenols with acetic anhydride at room temperature: Synth. Commun., 28, 3173 (1998). Solvent-free tetrahydropyranylation of alcohols has been accomplished under mild conditions: Synth. Commun., 33, 3929 (2003). Catalyzes the addition-esterification of aliphatic carboxylic acids with cyclic olefins: Catal. Lett., 96, 71 (2004). For a brief feature on uses in synthesis, see: Synlett, 1342 (2005).
- Kamal, A.; Babu, K. S.; Hussaini, S. M. A.; Srikanth, p. S.; Balakrishna, M.; Alarifi, A. Sulfamic acid: an efficient and recyclable solid acid catalyst for the synthesis of 4,5-dihydropyrrolo[1,2-a]quinoxalines. Tetrahedron Lett. 2015, 56 (31), 4619-4622.
- Kamal, A.; Babu, K. S.; Vardhan, M. V. P. S. V.; Hussaini, S. M. A.; Mahesh, R.; Shaik, S. P.; Alarifi, A. Sulfamic acid promoted one-pot three-component synthesis and cytotoxic evaluation of spirooxindoles. Bioorg. Med. Chem. Lett. 2015, 25 (10), 2199-2202.