Search Thermo Fisher Scientific
Thermo Scientific Chemicals
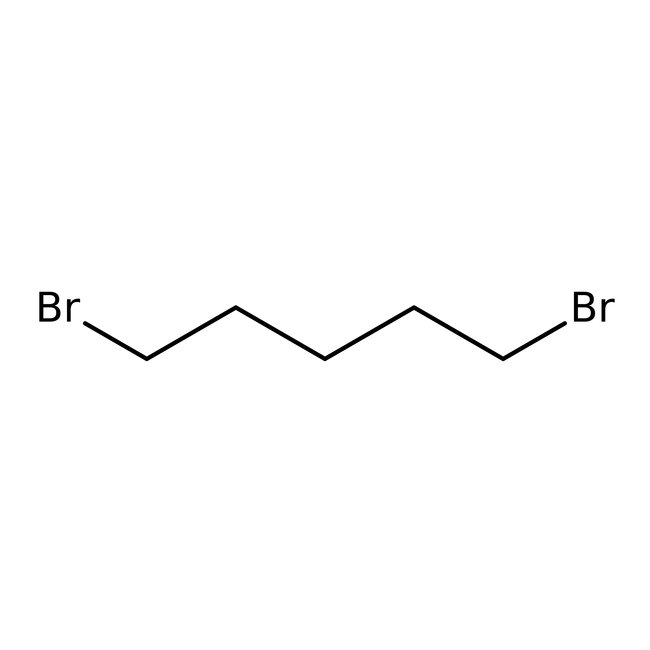
1,5-Dibromopentane, 98%, Thermo Scientific Chemicals
CAS: 111-24-0 | C5H10Br2 | 229.943 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFA18465.22 | 100 g |
Catalog number ALFA18465.22
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
100 g
Specifications
Chemical Name or Material1,5-Dibromopentane
CAS111-24-0
Health Hazard 1H227-H302-H315-H319
Health Hazard 2GHS H Statement
H315-H319-H335-H227
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
Combustible liquid.
H315-H319-H335-H227
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
Combustible liquid.
Health Hazard 3P210-P264b-P270-P280-P301+P312-P302+P352-P305+P351+P338-P330-P332+P313-P362-P370+P378q-P501c
View more
1,5-Dibromopentane is used in the preparation of 1,5-di-Grignard reagent and novel spirocyclic pyrrolidones. It acts as a growth supplement for Yarrowia lipolytica 3589, a tropical marine yeast. It is involved in the synthesis of N-alkylated piperidine as well as tetrydrothiopyran (thiane) by reaction with primary amine and sodium sulfide respectively.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
1,5-Dibromopentane is used in the preparation of 1,5-di-Grignard reagent and novel spirocyclic pyrrolidones. It acts as a growth supplement for Yarrowia lipolytica 3589, a tropical marine yeast. It is involved in the synthesis of N-alkylated piperidine as well as tetrydrothiopyran (thiane) by reaction with primary amine and sodium sulfide respectively.
Solubility
Miscible with water, slightly miscible with chloroform and benzene.
Notes
Incompatible with strong oxidizing agents and strong bases.
1,5-Dibromopentane is used in the preparation of 1,5-di-Grignard reagent and novel spirocyclic pyrrolidones. It acts as a growth supplement for Yarrowia lipolytica 3589, a tropical marine yeast. It is involved in the synthesis of N-alkylated piperidine as well as tetrydrothiopyran (thiane) by reaction with primary amine and sodium sulfide respectively.
Solubility
Miscible with water, slightly miscible with chloroform and benzene.
Notes
Incompatible with strong oxidizing agents and strong bases.
RUO – Research Use Only
General References:
- Lifa, T.; Tieu, W.; Hocking, R. K.; Codd, R. Forward and Reverse (Retro) Iron(III) or Gallium(III) Desferrioxamine E and Ring-Expanded Analogues Prepared Using Metal-Templated Synthesis from endo-Hydroxamic Acid Monomers. Inorg. Chem. 2015, 54 (7), 3573-3583.
- Perroni, D. V.; Baez-Cotto, C. M.; Sorenson, G. P.; Mahanthappa, M. K. Linker Length-Dependent Control of Gemini Surfactant Aqueous Lyotropic Gyroid Phase Stability. J. Phys. Chem. Lett. 2015, 6 (6), 993-998.