Search Thermo Fisher Scientific
Thermo Scientific Chemicals
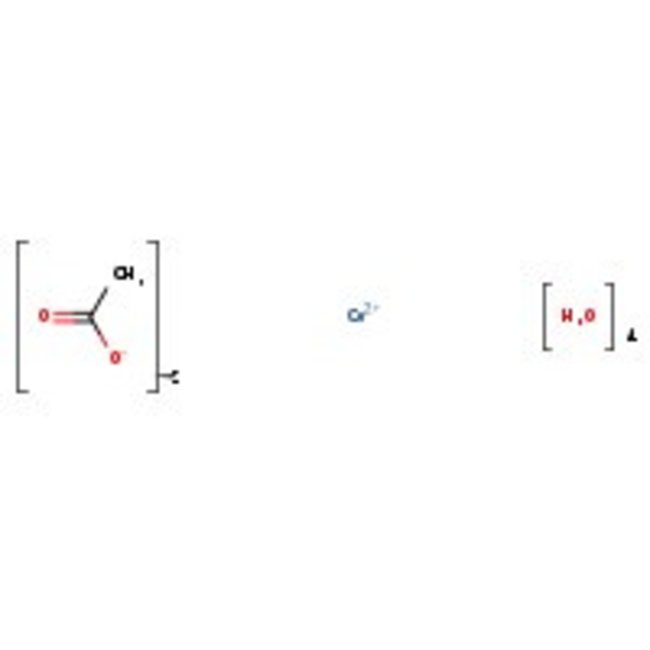
Cobalt(II) acetate tetrahydrate, 98%, Thermo Scientific Chemicals
Catalog number ALFA18374.36
Price (MYR)
854.00
Quantity:
500 g
Price (MYR)
854.00
Specifications
Chemical Name or MaterialCobalt(II) acetate tetrahydrate
CAS6147-53-1
Health Hazard 1H302-H317-H334-H335-H341-H350i-H360F
Health Hazard 2GHS H Statement
H334-H350-H360-H341-H302-H317
May cause allergy or asthma symptoms or breathing difficulties if inhaled.
May cause cancer.
May damage fertility or the unborn child.
Suspected of causing genetic defects.
Harmful if swallowed.
May cause an allergic skin reaction.
H334-H350-H360-H341-H302-H317
May cause allergy or asthma symptoms or breathing difficulties if inhaled.
May cause cancer.
May damage fertility or the unborn child.
Suspected of causing genetic defects.
Harmful if swallowed.
May cause an allergic skin reaction.
Health Hazard 3P201-P202-P261-P271-P272-P280g-P281-P285-P302+P352-P304+P340-P308+P313-P333+P313-P342+P311-P363-P501c
View more
Cobalt(II) acetate tetrahydrate is used as an industrial catalyst to harden paints and varnishes, an active catalyst for oxidation and esterification reactions. It is also used to prepare metal complexes having unusual coordination geometry.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Cobalt(II) acetate tetrahydrate is used as an industrial catalyst to harden paints and varnishes, an active catalyst for oxidation and esterification reactions. It is also used to prepare metal complexes having unusual coordination geometry.
Solubility
Soluble in water, alcohol, dilute acids and phenyl acetate.
Notes
Keep container tightly closed in a dry and well-ventilated place. Incompatible with strong acids and bases.
Cobalt(II) acetate tetrahydrate is used as an industrial catalyst to harden paints and varnishes, an active catalyst for oxidation and esterification reactions. It is also used to prepare metal complexes having unusual coordination geometry.
Solubility
Soluble in water, alcohol, dilute acids and phenyl acetate.
Notes
Keep container tightly closed in a dry and well-ventilated place. Incompatible with strong acids and bases.
RUO – Research Use Only
General References:
- Jang, W.; Kim, S. E.; Yang, C. M.; Yoon, S.; Park, M.; Lee, J.; Kim, Y.; Kim, M. Cobaltitrophenolate-catalyzed selective conversion of aldoximes into nitriles or amides. Catal. Commun. 2015, 60, 120-123.
- Wang, P.; Zhao, L. Synthesis, structure and spectroscopic properties of the trinuclear cobalt(II) and nickel(II) complexes based on 2-hydroxynaphthaldehyde and bis(aminooxy)alkane. Spectrochim. Acta, Part A 2015, 135, 342-350.