Search Thermo Fisher Scientific
Thermo Scientific Chemicals
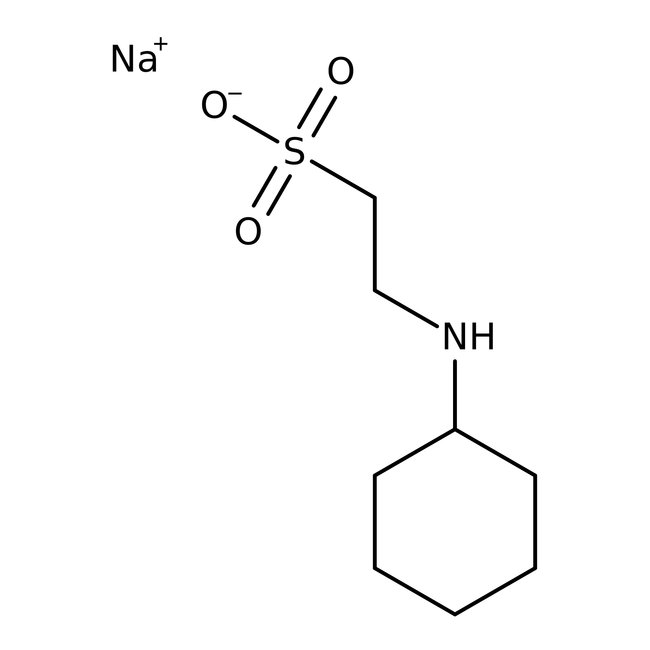
CHES, 99%
CAS: 103-47-9 | C8H17NO3S | 207.288 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFA18047.14 | 25 g |
Catalog number ALFA18047.14
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
25 g
Chemical Identifiers
CAS7051-34-5
IUPAC Name(bromomethyl)cyclopropane
Molecular FormulaC4H7Br
InChI KeyAEILLAXRDHDKDY-UHFFFAOYSA-N
SMILESBrCC1CC1
View more
Specifications Specification Sheet
Specification Sheet
Appearance (Color)Clear colorless to slightly colored
Water=<0.1 % (K.F.)
Specific gravity(20°C) 1.385 to 1.415
Infrared spectrumConforms
Appearance (Form)Liquid
View more
CHES is zwitterionic buffer useful in pH range 8.6 to 10.0.CHES is widely utilized as a buffer which is used for investigations on pH-dependent processes in enzymology. It has been shown to have an unusually high affinity for the iodoacetate binding site of liver alcohol dehydrogenase.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
CHES is zwitterionic buffer useful in pH range 8.6 to 10.0.CHES is widely utilized as a buffer which is used for investigations on pH-dependent processes in enzymology. It has been shown to have an unusually high affinity for the iodoacetate binding site of liver alcohol dehydrogenase.
Solubility
Soluble in water.
Notes
Store in cool place. Hygroscopic. Incompatible with strong oxidizing agents.
CHES is zwitterionic buffer useful in pH range 8.6 to 10.0.CHES is widely utilized as a buffer which is used for investigations on pH-dependent processes in enzymology. It has been shown to have an unusually high affinity for the iodoacetate binding site of liver alcohol dehydrogenase.
Solubility
Soluble in water.
Notes
Store in cool place. Hygroscopic. Incompatible with strong oxidizing agents.
RUO – Research Use Only
General References:
- Biological buffer, pKa = 9.3 at 20°: Biochemistry, 5, 467 (1966).
- Ferreira, C. M. H.; Pinto, I. S. S.; Soares, E. V.; Soares, H. M. V. M. (Un)suitability of the use of pH buffers in biological, biochemical and environmental studies and their interaction with metal ions - a review. RSC Adv. 2015, 5 (39), 30989-31003.
- Devi, T. S. R.; kumar, J. S.; Ramkumaar, G. R. DFT analysis on the molecular structure, vibrational and electronic spectra of 2-(cyclohexylamino)ethanesulfonic acid. Spectrochim. Acta, Part A 2015, 137, 761-777.