Search Thermo Fisher Scientific
Thermo Scientific Chemicals
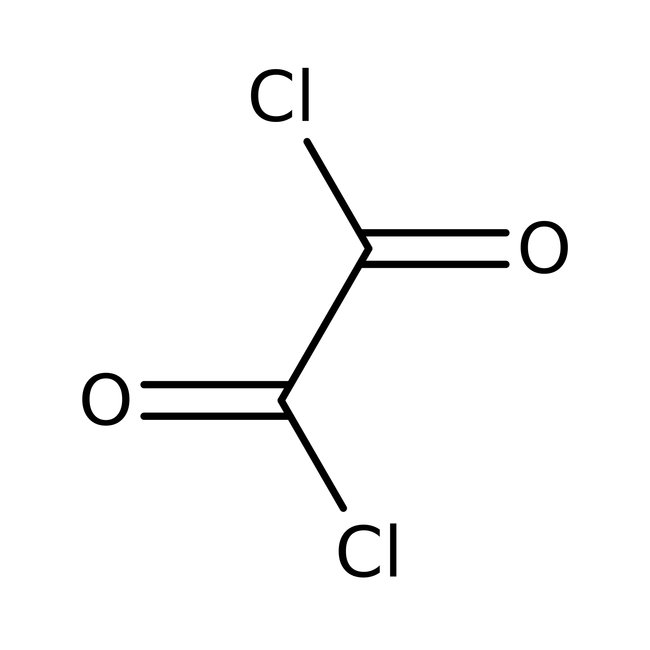
Oxalyl chloride, 98%, Thermo Scientific Chemicals
CAS: 79-37-8 | C2Cl2O2 | 126.92 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFA18012.18 | 50 g |
Catalog number ALFA18012.18
Price (MYR)
357.00
Quantity:
50 g
Price (MYR)
357.00
Specifications
Chemical Name or MaterialOxalyl chloride
CAS79-37-8
Health Hazard 1H260-H301+H331-H314
Health Hazard 2GHS H Statement
H331-H314-H318-H302
H331-H314-H318-H302
Health Hazard 3P223-P231+P232-P260-P264b-P270-P271-P280-P284-P303+P361+P353-P304+P340-P305+P351+P338-P310-P330-P331-P335+P334-P363-P370+P378q-P501c
View more
Ammonium hexafluorotitanate is used as an anti-corrosive cleaning agent. It is also used for the production of ceramics and glass. Further, it is used in the preparation of synthetic gems.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Suitable for battery materials development.
Applications
Oxalyl chloride is mainly used as a catalyst in the oxidation of alcohols to the corresponding aldehydes and ketones. It is actively used for the synthesis of acid chlorides from acids. In the Fridel-Crafts acylation reaction, it reacts with aromatic compounds to get the corresponding acyl choride using aluminum chloride as catalyst. Further, it is utilized to prepare dioxane tetrketone, an oxide of carbon.
Solubility
Oxalyl chloride Slightly miscible with water.Miscible with ether, benzene and chloroform.
Notes
Moisture sensitive. Incompatible with bases, alcohols, water, amines and metals.
Oxalyl chloride is mainly used as a catalyst in the oxidation of alcohols to the corresponding aldehydes and ketones. It is actively used for the synthesis of acid chlorides from acids. In the Fridel-Crafts acylation reaction, it reacts with aromatic compounds to get the corresponding acyl choride using aluminum chloride as catalyst. Further, it is utilized to prepare dioxane tetrketone, an oxide of carbon.
Solubility
Oxalyl chloride Slightly miscible with water.Miscible with ether, benzene and chloroform.
Notes
Moisture sensitive. Incompatible with bases, alcohols, water, amines and metals.
RUO – Research Use Only
General References:
- Reactive acid chloride which can be used as a phosgene substitute in many reactions.
- Caution! Carbon monoxide may be evolved.
- Mild reagent for conversion of sensitive acids to acid chlorides; see, e.g.: Org. Synth. Coll., 8, 486 (1993); Org. Synth. Coll., 9, 516 (1998).
- Has advantages over POCl3 in the Vilsmeier formylation reaction in that cleaner reactions often occur and a much lower mass of acidic by-product is formed. See N,N-Dimethyl formamide, A13547 and (Chloromethyl ene) dimethyl ammonium chloride, B24172.
- Friedel-Crafts reaction with arenes give carboxylic acids via the acid chlorides; see, e.g.: Org. Synth. Coll., 5, 706 (1973); 7, 420 (1990). For other phosgene equivalents, see Trichloromethyl chloroformate, A17444, and Triphosgene, A14932.
- Widely used to activate Dimethyl sulfoxide, A13280, for selective oxidation of alcohols to aldehydes or ketones (Swern oxidation) at low temperatures under very mild conditions: J. Org. Chem., 43, 2480 (1978). The reactive species is thought to be chlorodimethylsulfonium chloride: J. Org. Chem., 44, 4148 (1979). For examples of Swern oxidations, see: Org. Synth. Coll., 7, 258 (1990); 8, 501 (1993); 9, 692 (1998); Org. Synth., 76, 110 (1998). See also Trifluoroacetic anhydride, A13614.
- Reaction with Grignards in the presence of LiBr and CuBr provides a route to symmetrical ɑ-diones in good yield: Tetrahedron Lett., 36, 7305 (1995).
- For a brief feature on uses of this reagent in Organic synthesis, see: Synlett, 1172 (2007).
- Ilangovan, A.; Anandhan, K.; Kaushik, M. P. Facile and selective deprotection of PMB ethers and esters using oxalyl chloride. Tetrahedron Lett. 2015, 56 (9), 1080-1084.
- Hansen, S. V.; Ulven, T. Oxalyl Chloride as a Practical Carbon Monoxide Source for Carbonylation Reactions. Org. Lett. 2015, 17 (11), 2832-2835.

.png-150.jpg)
