Search Thermo Fisher Scientific
Thermo Scientific Chemicals
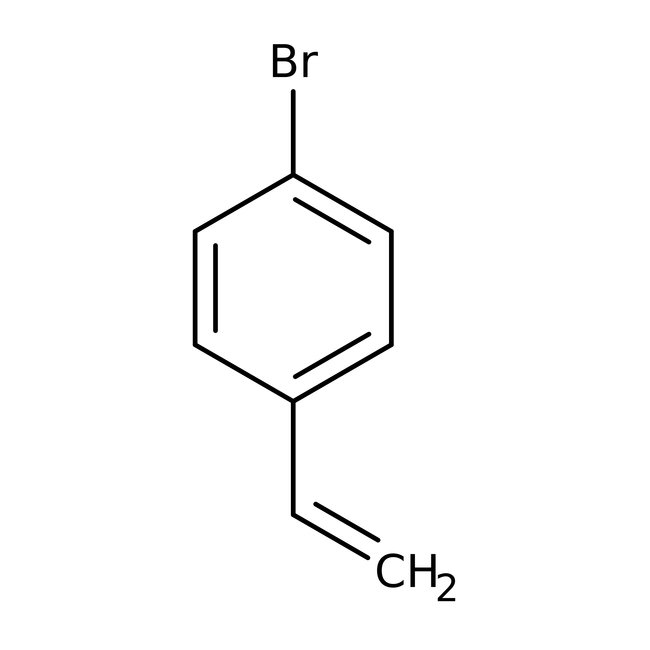
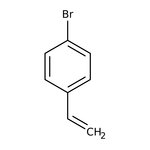
4-Bromostyrene, 98%, stab. with 0.1% 4-tert-butylcatechol, Thermo Scientific Chemicals
CAS: 2039-82-9 | C8H7Br | 183.05 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFA17896.22 | 100 g |
Catalog number ALFA17896.22
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
100 g
Chemical Identifiers
CAS67-63-0
IUPAC Namepropan-2-ol
Molecular FormulaC3H8O
InChI KeyKFZMGEQAYNKOFK-UHFFFAOYSA-N
SMILESCC(C)O
View more
Specifications Specification Sheet
Specification Sheet
Specific gravity(25°C) 0.783 to 0.787
Identification DPasses test
Non-volatile matter=<20 ppm
Water=<0.5 %
UVat 290 nm A: =<0.02
View more
4-Bromostyrene is widely utilized in the synthesis of nitroolefins by alkene cross-metathesis. It plays an important role in the Heck reaction to the synthesis of poly(1,4-phenylenevinylene). It is employed in the structure activity relationships (SAR) study of the chemical and biochemical properties of the vinyl group of styrene. It is also used to investigate the photochemical growth of Br-terminated self-assembled monolayers. It is also involved in the synthesis of silsesquioxanes.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
4-Bromostyrene is widely utilized in the synthesis of nitroolefins by alkene cross-metathesis. It plays an important role in the Heck reaction to the synthesis of poly(1,4-phenylenevinylene). It is employed in the structure activity relationships (SAR) study of the chemical and biochemical properties of the vinyl group of styrene. It is also used to investigate the photochemical growth of Br-terminated self-assembled monolayers. It is also involved in the synthesis of silsesquioxanes.
Solubility
Miscible with ethanol, ether and benzene.
Notes
Store in a cool place. Keep the container tightly closed in a dry and well-ventilated place. Incompatible with acids, bases, oxidizing agents and halogens. Keep away from sources of ignition.
4-Bromostyrene is widely utilized in the synthesis of nitroolefins by alkene cross-metathesis. It plays an important role in the Heck reaction to the synthesis of poly(1,4-phenylenevinylene). It is employed in the structure activity relationships (SAR) study of the chemical and biochemical properties of the vinyl group of styrene. It is also used to investigate the photochemical growth of Br-terminated self-assembled monolayers. It is also involved in the synthesis of silsesquioxanes.
Solubility
Miscible with ethanol, ether and benzene.
Notes
Store in a cool place. Keep the container tightly closed in a dry and well-ventilated place. Incompatible with acids, bases, oxidizing agents and halogens. Keep away from sources of ignition.
RUO – Research Use Only
General References:
- Zhang, Y. P.; He, J. H.; Xu, G. Q. Selective Attachment of 4-Bromostyrene on the Si(111)-(7 x 7) Surface. J. Phys. Chem. C 2011, 115 (31), 15496-15501.
- Demirel, G. B.; Çakmak, M.; Çaykara, T. Understanding 4-bromostyrene Adsorption on the Si(001)-(1 x 2) surface: A density functional theory study. Surf. Sci. 2011, 605 (11-12), 1056-1061.