Search Thermo Fisher Scientific
Thermo Scientific Chemicals
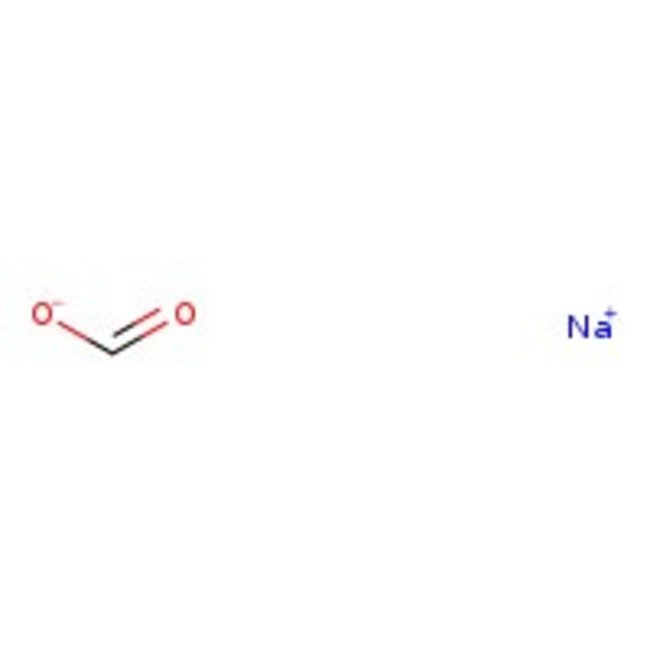
Sodium formate, 98%, Thermo Scientific Chemicals
CAS: 141-53-7 | CHNaO2 | 68.007 g/mol
Catalog number ALFA17813.0B
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
1000 g
Specifications
Chemical Name or MaterialSodium formate
CAS141-53-7
Health Hazard 1H315-H320-H335-H500
Health Hazard 3P210-P261-P264b-P271-P280-P302+P352-P304+P340-P305+P351+P338-P312-P332+P313-P362-P501c
Melting Point259°C to 262°C
View more
Sodium formate is used in analytical chemistry to precipitate noble metals. It is used for tanning leather, for dyeing and printing fabrics. It acts as an electroplating agent, an acidulant in the textile industry, a reducing agent, a mordant, a complexing agent. Further, it is used in photographic fixing baths. In addition, it is used as a buffering agent for strong mineral acids and food additive.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Sodium formate is used in analytical chemistry to precipitate noble metals. It is used for tanning leather, for dyeing and printing fabrics. It acts as an electroplating agent, an acidulant in the textile industry, a reducing agent, a mordant, a complexing agent. Further, it is used in photographic fixing baths. In addition, it is used as a buffering agent for strong mineral acids and food additive.
Solubility
Soluble in water, alcohol, glycerol and formic acid. Insoluble in ether.
Notes
Hygroscopic. Incompatible with strong oxidizing agents, strong acids. Moisture sensitive
Sodium formate is used in analytical chemistry to precipitate noble metals. It is used for tanning leather, for dyeing and printing fabrics. It acts as an electroplating agent, an acidulant in the textile industry, a reducing agent, a mordant, a complexing agent. Further, it is used in photographic fixing baths. In addition, it is used as a buffering agent for strong mineral acids and food additive.
Solubility
Soluble in water, alcohol, glycerol and formic acid. Insoluble in ether.
Notes
Hygroscopic. Incompatible with strong oxidizing agents, strong acids. Moisture sensitive
RUO – Research Use Only
General References:
- Converts Grignard reagents to aldehydes in excellent yields: Tetrahedron Lett., 25, 1843 (1984). Lithium formate may also be used.
- Under phase-transfer conditions converts primary alkyl chlorides to alcohols via the formate esters: Synthesis, 763 (1986).
- Reaction with acetyl chloride gives the useful formylating agent, formic acetic anhydride. For details, including isolation by distillation, see: Org. Synth. Coll., 6, 8 (1988); review: Tetrahedron, 46, 1081 (1990).
- Reduces amine oxides to amines under mild conditions: Chem. Lett., 1517 (1985).
- Can be used as a hydrogen donor in catalytic transfer hydrogenation reactions. More active hydrogen donor than formic acid in hydrogenolysis of aryl chlorides. For a comparative mechanistic study of formic acid and formate salts in this reaction, see: J. Org. Chem., 60, 1347 (1995). See also Ammonium formate, A10699 .
- Kathó, Á.; Szatmári, I.; Papp, G.; Joó, F. Effect of 2-Propanol on the Transfer Hydrogenation of Aldehydes by Aqueous Sodium Formate using a Rhodium(I)-sulfonated Triphenylphosphine Catalyst. Int. J. Chem. 2015, 69 (6), 339-344.
- Soni, R.; Hall, T. H.; Mitchell, B. P.; Owen, M. R.; Wills, M. Asymmetric reduction of electron-rich ketones with tethered ru(II)/TsDPEN catalysts using formic acid/triethylamine or aqueous sodium formate. J. Org. Chem. 2015, 80 (13), 6784-6793.