Search Thermo Fisher Scientific
Perfluoro-n-octane, 98%, Thermo Scientific Chemicals
Catalog number ALFA17741.06
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
5 g
Specifications
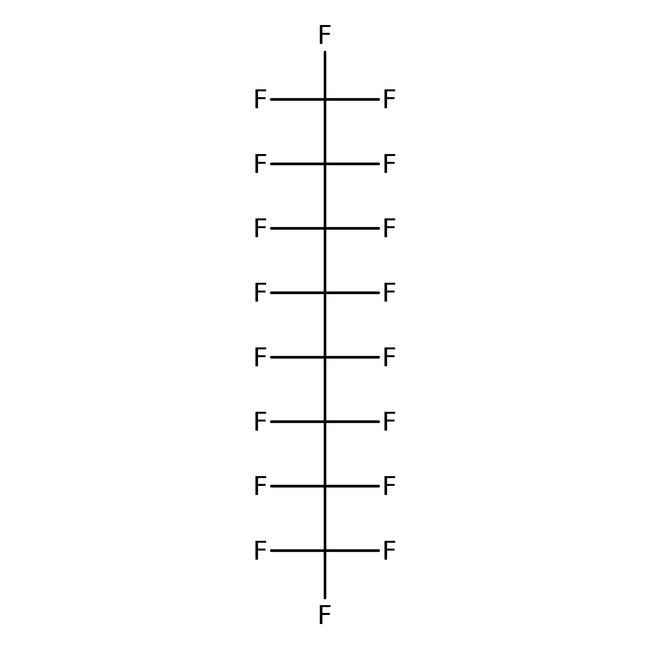
Chemical Name or MaterialPerfluoro-n-octane
CAS307-34-6
Recommended StorageAmbient temperatures
Density1.73
RTECS NumberRG9701000
View more
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Perfluorooctane may be used in chemical synthesis.
Solubility
Not miscible or difficult to mix in water.
Notes
Store away from oxidizing agents. Keep the container tightly closed in a cool, dry and well ventilated place.
Perfluorooctane may be used in chemical synthesis.
Solubility
Not miscible or difficult to mix in water.
Notes
Store away from oxidizing agents. Keep the container tightly closed in a cool, dry and well ventilated place.
RUO – Research Use Only
General References:
- Cathrine Carlsen Bach.; Bodil Hammer Bech.; Nis Brix.; Ellen Aagaard Nohr.; Jens Peter Ellekilde Bonde.; Tine Brink Henriksen. Perfluoroalkyl and polyfluoroalkyl substances and human fetal growth: A systematic review.Crit Rev Toxicol.2015,45(1), 53-67.
- Lo Nostro P and Chen SH. Aggregation of a semifluorinated n-alkane in perfluorooctane. J. Phys. Chem.,1993,97(24), 6535-40.
- James E Klaunig.; Motoki Shinohara.; Hiroyuki Iwai.; Christopher P Chengelis.; Jeannie B Kirkpatrick.; Zemin Wang.; Richard H Bruner. Evaluation of the chronic toxicity and carcinogenicity of perfluorohexanoic acid (PFHxA) in Sprague-Dawley rats.Toxicol Pathol.,2015,43(2), 209-220.
- Reaction medium with substantial capacity to solubilize molecular oxygen as in the photooxidation of alkenes: Synth. Commun., 26, 1861 (1996), or the high yield epoxidation of alkenes in the presence of trimethylacetaldehyde: Synth. Commun., 27, 447 (1997).