Search Thermo Fisher Scientific
Thermo Scientific Chemicals
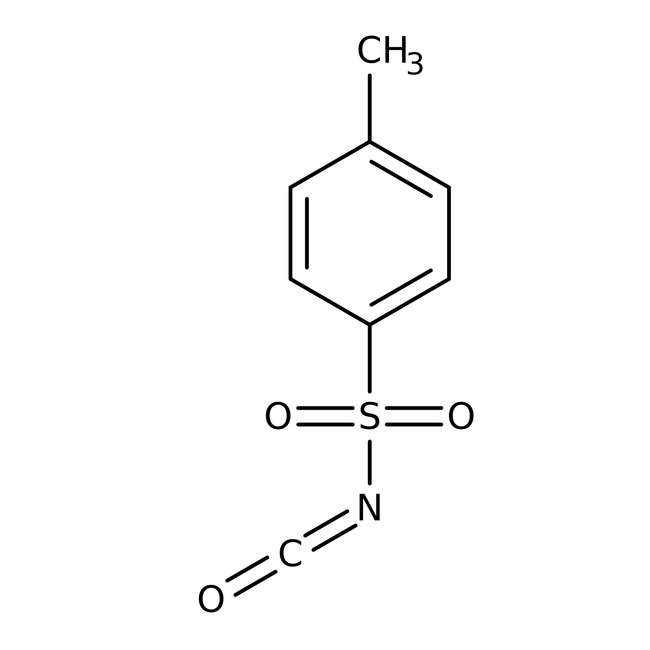
p-Toluenesulfonyl isocyanate, 95%, Thermo Scientific Chemicals
CAS: 4083-64-1 | C8H7NO3S | 197.21 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFA17492.14 | 25 g |
Catalog number ALFA17492.14
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
25 g
Chemical Identifiers
CAS2142-63-4
IUPAC Name1-(3-bromophenyl)ethan-1-one
Molecular FormulaC8H7BrO
InChI KeyJYAQYXOVOHJRCS-UHFFFAOYSA-N
SMILESCC(=O)C1=CC=CC(Br)=C1
View more
Specifications Specification Sheet
Specification Sheet
Refractive index1.5749 to 1.5769 (20°C, 589 nm)
Appearance (Color)Clear colorless to yellow
Appearance (Form)Liquid
Infrared spectrumConforms
GC>=96.0 %
It is a reagent used to prepare acetylated syn-1,2-diols,oxazolidin-2-ones, 2,3-diamino acids, and N-tosylcarbonamides. -Toluenesulfonyl isocyanate has been used as derivatization reagent in determination of 3-α-hydroxy tibolone in human plasma by LC-MS/MS. It is also employed in the derivatization reagent in simultaneous quantitative analysis of diethylene glycol and propylene glycol in pharmaceutical products by HPLC and as reagent in the preparation of acetylated syn-1,2-diols, oxazolidin-2-ones, 2,3-diamino acids and N-tosylcarbonamides.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
It is a reagent used to prepare acetylated syn-1,2-diols,oxazolidin-2-ones, 2,3-diamino acids, and N-tosylcarbonamides. -Toluenesulfonyl isocyanate has been used as derivatization reagent in determination of 3-α-hydroxy tibolone in human plasma by LC-MS/MS. It is also employed in the derivatization reagent in simultaneous quantitative analysis of diethylene glycol and propylene glycol in pharmaceutical products by HPLC and as reagent in the preparation of acetylated syn-1,2-diols, oxazolidin-2-ones, 2,3-diamino acids and N-tosylcarbonamides.
Solubility
Reacts with water.
Notes
Keep away from alcohols, strong bases, amines, strong oxidizing agents.
It is a reagent used to prepare acetylated syn-1,2-diols,oxazolidin-2-ones, 2,3-diamino acids, and N-tosylcarbonamides. -Toluenesulfonyl isocyanate has been used as derivatization reagent in determination of 3-α-hydroxy tibolone in human plasma by LC-MS/MS. It is also employed in the derivatization reagent in simultaneous quantitative analysis of diethylene glycol and propylene glycol in pharmaceutical products by HPLC and as reagent in the preparation of acetylated syn-1,2-diols, oxazolidin-2-ones, 2,3-diamino acids and N-tosylcarbonamides.
Solubility
Reacts with water.
Notes
Keep away from alcohols, strong bases, amines, strong oxidizing agents.
RUO – Research Use Only
General References:
- C. King. Some Reactions of p-Toluenesulfonyl Isocyanate. J. Org. Chem. 1960, 25 (3),352-356.
- Priscilla B. Bell.; Andrew. Wojcicki. Kinetic study of cycloaddition reactions of transition metal-propargyl and -.eta.1-allyl complexes with p-toluenesulfonyl isocyanate. Inorg. Chem. 1981, 20 (5),1585-1592.
- Highly reactive isocyanate. For a brief feature on uses of the reagent in synthesis, see: Synlett, 2644 (2004).