Search Thermo Fisher Scientific
Thermo Scientific Chemicals
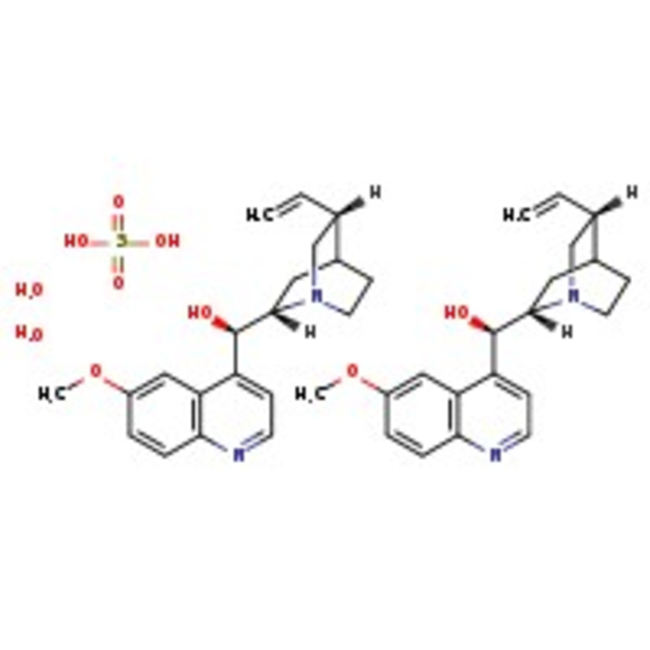
Quinine hemisulfate monohydrate, 98+%, Thermo Scientific Chemicals
CAS: 6119-70-6 | C40H54N4O10S | 782.95 g/mol
Catalog number ALFA17036.18
Quantity:
50 g
Chemical Identifiers
CAS77771-02-9
IUPAC Name3-bromo-4-fluorobenzaldehyde
Molecular FormulaC7H4BrFO
InChI KeyFAHZIKXYYRGSHF-UHFFFAOYSA-N
SMILESFC1=CC=C(C=O)C=C1Br
View more
Specifications Specification Sheet
Specification Sheet
Appearance (Color)White to light yellow
Appearance (Form)Low melting solid
Melting point28°C to 33°C
Infrared spectrumConforms
GC>=98.5 %
Quinine hemisulfate monohydrate plays a major role in potassium channel blockers. It is also used as an antimalarial, anticholinergic, antihypertensive and a hypoglycemic agent. It inhibits mitochondrial ATP-regulated potassium channel. It is also used to study the metabolism of biocrystalized heme, hemozoin, in malarial parasites and to study the toxicity of heme (FP)-complexes.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Quinine hemisulfate monohydrate plays a major role in potassium channel blockers. It is also used as an antimalarial, anticholinergic, antihypertensive and a hypoglycemic agent. It inhibits mitochondrial ATP-regulated potassium channel. It is also used to study the metabolism of biocrystalized heme, hemozoin, in malarial parasites and to study the toxicity of heme (FP)-complexes.
Solubility
Soluble in a mixture of chloroform and absolute alcohol (2:1).
Notes
Light sensitive. Incompatible with strong oxidizing agents, alkalis, ammonia, strong bases and iodine.
Quinine hemisulfate monohydrate plays a major role in potassium channel blockers. It is also used as an antimalarial, anticholinergic, antihypertensive and a hypoglycemic agent. It inhibits mitochondrial ATP-regulated potassium channel. It is also used to study the metabolism of biocrystalized heme, hemozoin, in malarial parasites and to study the toxicity of heme (FP)-complexes.
Solubility
Soluble in a mixture of chloroform and absolute alcohol (2:1).
Notes
Light sensitive. Incompatible with strong oxidizing agents, alkalis, ammonia, strong bases and iodine.
RUO – Research Use Only
General References:
- Krzeszewski, M.; Gryko, D. T. x-Shaped Bis (areno)-1, 4-dihydropyrrolo [3, 2-b] pyrroles Generated by Oxidative Aromatic Coupling. J. Org. Chem. 2015, 80 (5), 2893-2899.
- Janiga, A.; Krzeszewski, M.; Gryko, D. T. Diindolo [2, 3-b: 2', 3'-f] pyrrolo [3, 2-b] pyrroles as Electron-Rich, Ladder-Type Fluorophores: Synthesis and Optical Properties. Chem. Asian J. 2015, 10 (1), 212-218.