Search Thermo Fisher Scientific
Thermo Scientific Chemicals
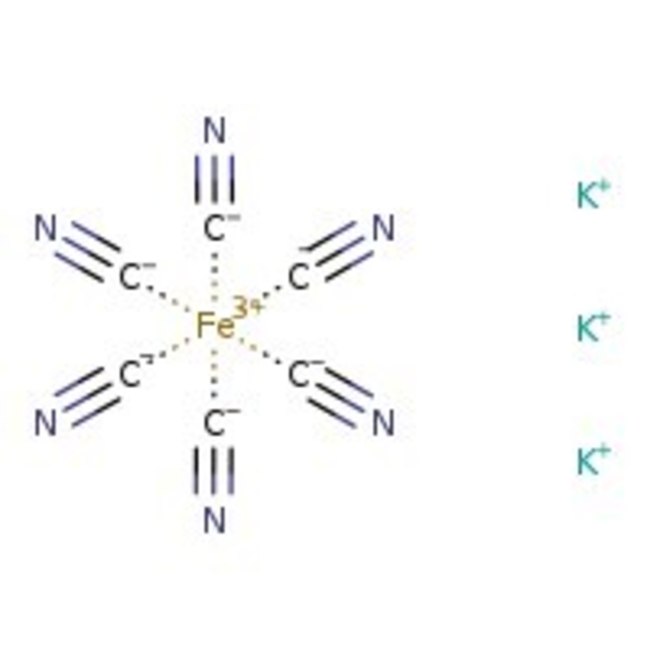
Potassium hexacyanoferrate(III), 98+%, Thermo Scientific Chemicals
CAS: 13746-66-2 | C6FeK3N6 | 329.25 g/mol
Catalog number ALFA16946.30
Price (MYR)
261.00
Quantity:
250 g
Price (MYR)
261.00
Specifications
Chemical Name or MaterialPotassium hexacyanoferrate(III)
Melting Point(decomposition)
CAS13746-66-2
Recommended StorageAmbient temperatures
RTECS NumberLJ8225000
View more
Potassium hexacyanoferrate(III) is used in reading palimpsests and old manuscripts, in blueprint drawing and in photography (Cyanotype process). It finds application to harden iron and steel, in electroplating, dyeing wool and as a laboratory reagent. It is a mild oxidizing agent used in organic synthesis. It is associated with potassium hydroxide solution used to formulate Murakami’s etchant, which is useful for metallographers to provide contrast between binder and carbide phases in cemented carbides. Further, it is used in many amperometric biosensors as an electron transfer agent replacing an enzyme's natural electron transfer agent such as oxygen with the enzyme glucose oxidase. It is an active ingredient in blood glucose meters used by diabetics.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Potassium hexacyanoferrate(III) is used in reading palimpsests and old manuscripts, in blueprint drawing and in photography (Cyanotype process). It finds application to harden iron and steel, in electroplating, dyeing wool and as a laboratory reagent. It is a mild oxidizing agent used in organic synthesis. It is associated with potassium hydroxide solution used to formulate Murakami’s etchant, which is useful for metallographers to provide contrast between binder and carbide phases in cemented carbides. Further, it is used in many amperometric biosensors as an electron transfer agent replacing an enzyme′s natural electron transfer agent such as oxygen with the enzyme glucose oxidase. It is an active ingredient in blood glucose meters used by diabetics.
Solubility
Soluble in water and acid. Slightly soluble in alcohol.
Notes
Light sensitive. Incompatible with strong acids, strong oxidizing agents, ammonia, hydrochloric acid and cyanides.
Potassium hexacyanoferrate(III) is used in reading palimpsests and old manuscripts, in blueprint drawing and in photography (Cyanotype process). It finds application to harden iron and steel, in electroplating, dyeing wool and as a laboratory reagent. It is a mild oxidizing agent used in organic synthesis. It is associated with potassium hydroxide solution used to formulate Murakami’s etchant, which is useful for metallographers to provide contrast between binder and carbide phases in cemented carbides. Further, it is used in many amperometric biosensors as an electron transfer agent replacing an enzyme′s natural electron transfer agent such as oxygen with the enzyme glucose oxidase. It is an active ingredient in blood glucose meters used by diabetics.
Solubility
Soluble in water and acid. Slightly soluble in alcohol.
Notes
Light sensitive. Incompatible with strong acids, strong oxidizing agents, ammonia, hydrochloric acid and cyanides.
RUO – Research Use Only
General References:
- do Carmo, D. R.; Silvestrini, D. R.; da Silveira, T. F.; Cumba, L. R.; Dias Filho, N. L.; Soares, L. A. Silsesquioxane organofunctionalized with 4-amino-3-hydrazino-5-mercapto-1,2,4-triazole: Preparation and subsequent reaction with silver and potassium hexacyanoferrate(III) for detection of l-cysteine. Mater. Sci. Eng., C 2015, 57, 24-30.
- Matsishin, M.; Rachkov, A.; Errachid, A.; Dzyadevych, S.; Soldatkin, A. Development of impedimetric DNA biosensor for selective detection and discrimination of oligonucleotide sequences of the rpoB gene of Mycobacterium tuberculosis. Sens. Actuators B Chem. 2015, 222, 1152-1158.

.png-150.jpg)
